In Vivo Methods to Monitor Cardiomyocyte Proliferation
- PMID: 35323621
- PMCID: PMC8950582
- DOI: 10.3390/jcdd9030073
In Vivo Methods to Monitor Cardiomyocyte Proliferation
Abstract
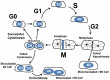
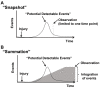
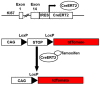
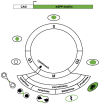
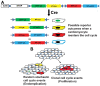
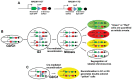
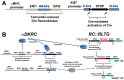
Adult mammalian cardiomyocytes demonstrate scarce cycling and even lower proliferation rates in response to injury. Signals that enhance cardiomyocyte proliferation after injury will be groundbreaking, address unmet clinical needs, and represent new strategies to treat cardiovascular diseases. In vivo methods to monitor cardiomyocyte proliferation are critical to addressing this challenge. Fortunately, advances in transgenic approaches provide sophisticated techniques to quantify cardiomyocyte cycling and proliferation.
Keywords: cardiomyocyte cycling; heart; mouse; regeneration; transgenics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Long Noncoding RNA CPR (Cardiomyocyte Proliferation Regulator) Regulates Cardiomyocyte Proliferation and Cardiac Repair.Circulation. 2019 Jun 4;139(23):2668-2684. doi: 10.1161/CIRCULATIONAHA.118.035832. Epub 2019 Mar 5. Circulation. 2019. PMID: 30832495
-
Stereological estimation of cardiomyocyte number and proliferation.Methods. 2021 Jun;190:55-62. doi: 10.1016/j.ymeth.2020.06.002. Epub 2020 Jun 27. Methods. 2021. PMID: 32603825
-
Cardiomyocyte cell cycle dynamics and proliferation revealed through cardiac-specific transgenesis of fluorescent ubiquitinated cell cycle indicator (FUCCI).J Mol Cell Cardiol. 2019 Feb;127:154-164. doi: 10.1016/j.yjmcc.2018.12.007. Epub 2018 Dec 18. J Mol Cell Cardiol. 2019. PMID: 30571978 Free PMC article.
-
Towards regenerating the mammalian heart: challenges in evaluating experimentally induced adult mammalian cardiomyocyte proliferation.Am J Physiol Heart Circ Physiol. 2016 May 1;310(9):H1045-54. doi: 10.1152/ajpheart.00697.2015. Epub 2016 Feb 26. Am J Physiol Heart Circ Physiol. 2016. PMID: 26921436 Review.
-
Cardiomyocyte proliferation in cardiac development and regeneration: a guide to methodologies and interpretations.Am J Physiol Heart Circ Physiol. 2015 Oct;309(8):H1237-50. doi: 10.1152/ajpheart.00559.2015. Epub 2015 Sep 4. Am J Physiol Heart Circ Physiol. 2015. PMID: 26342071 Review.
References
-
- Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Das S.R., et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659. - DOI - PubMed
-
- Yancy C.W., Jessup M., Bozkurt B., Butler J., Casey D.E., Jr., Drazner M.H., Fonarow G.C., Geraci S.A., Horwich T., Januzzi J.L., et al. 2013 ACCF/AHA guideline for the management of heart failure: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–1852. doi: 10.1161/CIR.0b013e31829e8807. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

