Inhibition of calcium-triggered secretion by hydrocarbon-stapled peptides
- PMID: 35322233
- PMCID: PMC8967716
- DOI: 10.1038/s41586-022-04543-1
Inhibition of calcium-triggered secretion by hydrocarbon-stapled peptides
Abstract
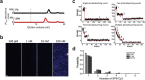
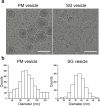
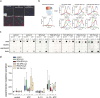
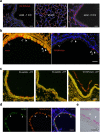
Membrane fusion triggered by Ca2+ is orchestrated by a conserved set of proteins to mediate synaptic neurotransmitter release, mucin secretion and other regulated exocytic processes1-4. For neurotransmitter release, the Ca2+ sensitivity is introduced by interactions between the Ca2+ sensor synaptotagmin and the SNARE complex5, and sequence conservation and functional studies suggest that this mechanism is also conserved for mucin secretion6. Disruption of Ca2+-triggered membrane fusion by a pharmacological agent would have therapeutic value for mucus hypersecretion as it is the major cause of airway obstruction in the pathophysiology of respiratory viral infection, asthma, chronic obstructive pulmonary disease and cystic fibrosis7-11. Here we designed a hydrocarbon-stapled peptide that specifically disrupts Ca2+-triggered membrane fusion by interfering with the so-called primary interface between the neuronal SNARE complex and the Ca2+-binding C2B domain of synaptotagmin-1. In reconstituted systems with these neuronal synaptic proteins or with their airway homologues syntaxin-3, SNAP-23, VAMP8, synaptotagmin-2, along with Munc13-2 and Munc18-2, the stapled peptide strongly suppressed Ca2+-triggered fusion at physiological Ca2+ concentrations. Conjugation of cell-penetrating peptides to the stapled peptide resulted in efficient delivery into cultured human airway epithelial cells and mouse airway epithelium, where it markedly and specifically reduced stimulated mucin secretion in both systems, and substantially attenuated mucus occlusion of mouse airways. Taken together, peptides that disrupt Ca2+-triggered membrane fusion may enable the therapeutic modulation of mucin secretory pathways.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Comment in
-
Mucus secretion blocked at its source in the lungs.Nature. 2022 Mar;603(7903):798-799. doi: 10.1038/d41586-022-00700-8. Nature. 2022. PMID: 35322214 No abstract available.
Similar articles
-
Screening of Hydrocarbon-Stapled Peptides for Inhibition of Calcium-Triggered Exocytosis.Front Pharmacol. 2022 Jun 17;13:891041. doi: 10.3389/fphar.2022.891041. eCollection 2022. Front Pharmacol. 2022. PMID: 35814209 Free PMC article.
-
Synaptotagmin-1-, Munc18-1-, and Munc13-1-dependent liposome fusion with a few neuronal SNAREs.Proc Natl Acad Sci U S A. 2021 Jan 26;118(4):e2019314118. doi: 10.1073/pnas.2019314118. Proc Natl Acad Sci U S A. 2021. PMID: 33468652 Free PMC article.
-
Molecular Mechanisms of Synaptic Vesicle Priming by Munc13 and Munc18.Neuron. 2017 Aug 2;95(3):591-607.e10. doi: 10.1016/j.neuron.2017.07.004. Neuron. 2017. PMID: 28772123 Free PMC article.
-
The Core Complex of the Ca2+-Triggered Presynaptic Fusion Machinery.J Mol Biol. 2023 Jan 15;435(1):167853. doi: 10.1016/j.jmb.2022.167853. Epub 2022 Oct 13. J Mol Biol. 2023. PMID: 36243149 Free PMC article. Review.
-
Regulated mucin secretion from airway epithelial cells.Front Endocrinol (Lausanne). 2013 Sep 18;4:129. doi: 10.3389/fendo.2013.00129. Front Endocrinol (Lausanne). 2013. PMID: 24065956 Free PMC article. Review.
Cited by
-
STING guides the STX17-SNAP29-VAMP8 complex assembly to control autophagy.Cell Insight. 2024 Feb 2;3(2):100147. doi: 10.1016/j.cellin.2024.100147. eCollection 2024 Apr. Cell Insight. 2024. PMID: 38344386 Free PMC article.
-
Towards a better mucolytic.Eur Respir J. 2023 May 25;61(5):2300619. doi: 10.1183/13993003.00619-2023. Print 2023 May. Eur Respir J. 2023. PMID: 37230504 Free PMC article. No abstract available.
-
SNARE Modulators and SNARE Mimetic Peptides.Biomolecules. 2022 Nov 29;12(12):1779. doi: 10.3390/biom12121779. Biomolecules. 2022. PMID: 36551207 Free PMC article. Review.
-
Tetraspanin-8 sequesters syntaxin-2 to control biphasic release propensity of mucin granules.Nat Commun. 2023 Jun 22;14(1):3710. doi: 10.1038/s41467-023-39277-9. Nat Commun. 2023. PMID: 37349283 Free PMC article.
-
Synergistic regulation of fusion pore opening and dilation by SNARE and synaptotagmin-1.J Mol Cell Biol. 2024 Sep 30;16(4):mjae011. doi: 10.1093/jmcb/mjae011. J Mol Cell Biol. 2024. PMID: 38444183 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

