Insights Into Mitochondrial Dynamics in Chlamydial Infection
- PMID: 35321312
- PMCID: PMC8936178
- DOI: 10.3389/fcimb.2022.835181
Insights Into Mitochondrial Dynamics in Chlamydial Infection
Abstract
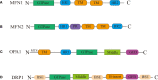
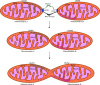
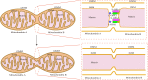
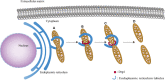
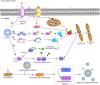
Mitochondria are intracellular organelles that are instrumental in the creation of energy, metabolism, apoptosis, and intrinsic immunity. Mitochondria exhibit an extraordinarily high degree of flexibility, and are constantly undergoing dynamic fusion and fission changes. Chlamydia is an intracellular bacterium that causes serious health problems in both humans and animals. Due to a deficiency of multiple metabolic enzymes, these pathogenic bacteria are highly dependent on their eukaryotic host cells, resulting in a close link between Chlamydia infection and host cell mitochondria. Indeed, Chlamydia increase mitochondrial fusion by inhibiting the activation of dynein-related protein 1 (DRP1), which can regulate host cell metabolism for extra energy. Additionally, Chlamydia can inhibit mitochondrial fission by blocking DRP1 oligomerization, preventing host cell apoptosis. These mechanisms are critical for maintaining a favorable environment for reproduction and growth of Chlamydia. This review discusses the molecular mechanisms of mitochondrial fusion and fission, as well as the mechanisms by which Chlamydia infection alters the mitochondrial dynamics and the prospects of limiting chlamydial development by altering mitochondrial dynamics.
Keywords: ATP; Chlamydia; DRP1; P53; mitochondrial dynamics.
Copyright © 2022 Yang, Lei, Zhao, Wen and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Chlamydia trachomatis Alters Mitochondrial Protein Composition and Secretes Effector Proteins That Target Mitochondria.mSphere. 2022 Dec 21;7(6):e0042322. doi: 10.1128/msphere.00423-22. Epub 2022 Oct 26. mSphere. 2022. PMID: 36286535 Free PMC article.
-
Chlamydia trachomatis targets mitochondrial dynamics to promote intracellular survival and proliferation.Cell Microbiol. 2019 Jan;21(1):e12962. doi: 10.1111/cmi.12962. Epub 2018 Oct 30. Cell Microbiol. 2019. PMID: 30311994
-
Chlamydia preserves the mitochondrial network necessary for replication via microRNA-dependent inhibition of fission.J Cell Biol. 2017 Apr 3;216(4):1071-1089. doi: 10.1083/jcb.201608063. Epub 2017 Mar 22. J Cell Biol. 2017. PMID: 28330939 Free PMC article.
-
The role of Drp1 adaptor proteins MiD49 and MiD51 in mitochondrial fission: implications for human disease.Clin Sci (Lond). 2016 Nov 1;130(21):1861-74. doi: 10.1042/CS20160030. Clin Sci (Lond). 2016. PMID: 27660309 Review.
-
[Mechanism of mitochondrial fission - structure and function of Drp1 protein].Postepy Biochem. 2016;62(2):127-137. Postepy Biochem. 2016. PMID: 28132464 Review. Polish.
Cited by
-
Modified Linggui Zhugan Decoction protects against ventricular remodeling through ameliorating mitochondrial damage in post-myocardial infarction rats.Front Cardiovasc Med. 2023 Jan 10;9:1038523. doi: 10.3389/fcvm.2022.1038523. eCollection 2022. Front Cardiovasc Med. 2023. PMID: 36704451 Free PMC article.
-
Amoebae as training grounds for microbial pathogens.mBio. 2024 Aug 14;15(8):e0082724. doi: 10.1128/mbio.00827-24. Epub 2024 Jul 8. mBio. 2024. PMID: 38975782 Free PMC article. Review.
-
Diabetes cardiomyopathy: targeted regulation of mitochondrial dysfunction and therapeutic potential of plant secondary metabolites.Front Pharmacol. 2024 Jul 9;15:1401961. doi: 10.3389/fphar.2024.1401961. eCollection 2024. Front Pharmacol. 2024. PMID: 39045049 Free PMC article. Review.
-
Chlamydia Infection Remodels Host Cell Mitochondria to Alter Energy Metabolism and Subvert Apoptosis.Microorganisms. 2023 May 24;11(6):1382. doi: 10.3390/microorganisms11061382. Microorganisms. 2023. PMID: 37374883 Free PMC article. Review.
-
Chlamydia trachomatis Alters Mitochondrial Protein Composition and Secretes Effector Proteins That Target Mitochondria.mSphere. 2022 Dec 21;7(6):e0042322. doi: 10.1128/msphere.00423-22. Epub 2022 Oct 26. mSphere. 2022. PMID: 36286535 Free PMC article.
References
-
- Abdul-Sater A. A., Saïd-Sadier N., Lam V. M., Singh B., Pettengill M. A., Soares F., et al. . (2010). Enhancement of Reactive Oxygen Species Production and Chlamydial Infection by the Mitochondrial Nod-Like Family Member NLRX1. J. Biol. Chem. 285 (53), 41637–41645. doi: 10.1074/jbc.M110.137885 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

