TIGIT Blockade Exerts Synergistic Effects on Microwave Ablation Against Cancer
- PMID: 35320940
- PMCID: PMC8935077
- DOI: 10.3389/fimmu.2022.832230
TIGIT Blockade Exerts Synergistic Effects on Microwave Ablation Against Cancer
Abstract
Background: Combination immunotherapy based on immune checkpoint inhibitors (ICIs) has shown great success in the treatment of many types of cancers and has become the mainstream in the comprehensive treatment of cancers. Ablation in combination with immunotherapy has achieved tremendous efficacy in some preclinical and clinical studies. To date, our team proved that ablation in combination with ICIs was a promising antitumor therapeutic strategy for the liver metastasis of colorectal cancer (CRC). Moreover, we found that the expression of T cell immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domain (TIGIT) expression was up-regulated after microwave ablation (MWA), indicating that TIGIT was involved in immunosuppression, and the combination of MWA and TIGIT blockade represented a potential clinical treatment strategy.
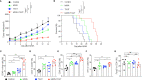
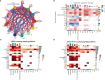
Methods: In the present study, we examined the expression of TIGIT using a preclinical mouse model treated with MWA. Moreover, we evaluated the antitumor functions of MWA alone or in combination with TIGIT blockade by monitoring tumor growth and survival of the mice. Besides, we also detected the numbers of tumor-infiltrating lymphocytes (TILs), and effector molecules of CD8+ T cells using flow cytometry. Finally, we analyzed the single-cell RNA sequencing (scRNA-seq) data from the MWA and MWA plus anti-TIGIT groups.
Results: The expression of TIGIT in various immune cells was up-regulated after MWA, and the addition of TIGIT blockade to MWA prolonged survival and delayed tumor growth in the MC38 tumor model. Taken together, our findings showed that TIGIT blockade in combination with MWA significantly promoted the expansion and functions of CD8+ TILs and reshaped myeloid cells in the tumor microenvironment (TME) using flow cytometry and scRNA-seq analysis.
Conclusions: TIGIT blockade in combination with MWA was a novel treatment strategy for the liver metastasis of CRC, and this combination therapy could reprogram the TME toward an antitumor environment.
Keywords: TIGIT; ablation; immune checkpoint inhibitors; immunotherapy; tumor microenvironment.
Copyright © 2022 Chen, Huang, Li, Xiao, Liu, Chen, Zhu, Zheng, Wu and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Microwave ablation combined with PD-L1 blockade synergistically promotes Cxcl9-mediated antitumor immunity.Cancer Sci. 2024 Jul;115(7):2196-2208. doi: 10.1111/cas.16182. Epub 2024 Apr 24. Cancer Sci. 2024. PMID: 38655660 Free PMC article.
-
LAG3 blockade coordinates with microwave ablation to promote CD8+ T cell-mediated anti-tumor immunity.J Transl Med. 2022 Sep 30;20(1):433. doi: 10.1186/s12967-022-03646-7. J Transl Med. 2022. PMID: 36180876 Free PMC article.
-
Combined use of microwave ablation and cell immunotherapy induces nonspecific immunity of hepatocellular carcinoma model mice.Cell Cycle. 2020 Dec;19(24):3595-3607. doi: 10.1080/15384101.2020.1853942. Epub 2020 Dec 7. Cell Cycle. 2020. PMID: 33283623 Free PMC article.
-
T-cell immunoglobulin and ITIM domain, as a potential immune checkpoint target for immunotherapy of colorectal cancer.IUBMB Life. 2021 May;73(5):726-738. doi: 10.1002/iub.2461. Epub 2021 Mar 30. IUBMB Life. 2021. PMID: 33686787 Review.
-
TIGIT, the Next Step Towards Successful Combination Immune Checkpoint Therapy in Cancer.Front Immunol. 2021 Jul 22;12:699895. doi: 10.3389/fimmu.2021.699895. eCollection 2021. Front Immunol. 2021. PMID: 34367161 Free PMC article. Review.
Cited by
-
Microwave ablation combined with PD-L1 blockade synergistically promotes Cxcl9-mediated antitumor immunity.Cancer Sci. 2024 Jul;115(7):2196-2208. doi: 10.1111/cas.16182. Epub 2024 Apr 24. Cancer Sci. 2024. PMID: 38655660 Free PMC article.
-
Development and evaluation of nanobody tracers for noninvasive nuclear imaging of the immune-checkpoint TIGIT.Front Immunol. 2023 Sep 20;14:1268900. doi: 10.3389/fimmu.2023.1268900. eCollection 2023. Front Immunol. 2023. PMID: 37799715 Free PMC article.
-
How Biology Guides the Combination of Locoregional Interventional Therapies and Immunotherapy for Hepatocellular Carcinoma: Cytokines and Their Roles.Cancers (Basel). 2023 Feb 19;15(4):1324. doi: 10.3390/cancers15041324. Cancers (Basel). 2023. PMID: 36831664 Free PMC article. Review.
-
Rational Nanomedicine Design Enhances Clinically Physical Treatment-Inspired or Combined Immunotherapy.Adv Sci (Weinh). 2022 Oct;9(29):e2203921. doi: 10.1002/advs.202203921. Epub 2022 Aug 24. Adv Sci (Weinh). 2022. PMID: 36002305 Free PMC article. Review.
-
Configuring Therapeutic Aspects of Immune Checkpoints in Lung Cancer.Cancers (Basel). 2023 Jan 16;15(2):543. doi: 10.3390/cancers15020543. Cancers (Basel). 2023. PMID: 36672492 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

