Feeder-Cell-Free and Serum-Free Expansion of Natural Killer Cells Using Cloudz Microspheres, G-Rex6M, and Human Platelet Lysate
- PMID: 35320938
- PMCID: PMC8934851
- DOI: 10.3389/fimmu.2022.803380
Feeder-Cell-Free and Serum-Free Expansion of Natural Killer Cells Using Cloudz Microspheres, G-Rex6M, and Human Platelet Lysate
Abstract
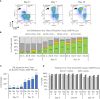
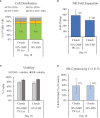
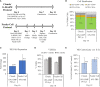
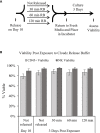
The versatility of natural killer cells has ignited growing interest in their therapeutic use for cancer and other immunotherapy treatments. However, NK cells compose a small portion of peripheral blood mononuclear cells (5%-20% of PBMCs) and clinical doses require billions of cells. Manufacturing suitable doses of NK cells remains a major challenge for NK immunotherapy. The current standard for expanding NK cells relies on feeder cells and fetal bovine serum to achieve large expansion, but both encounter regulatory concerns. We developed NK Cloudz, a dissolvable polymer-based microsphere platform, as an alternative to a feeder cell approach to expand NK cells. We demonstrated that a combination of NK Cloudz, a G-Rex6M culture vessel, and GMP Human Platelet Lysate expanded NK cells 387 ± 100-fold in 10 days from a PBMC starting population. The NK purity, viability, and cytotoxicity were similar to both a feeder cell protocol and an FBS-based protocol. Additionally, we found no significant differences between FBS and GMP Human Platelet Lysate and concluded that platelet lysate is a good xeno-free alternative to FBS for NK expansion. Overall, we demonstrated a feeder-cell-free and FBS-free protocol that leverages NK Cloudz as a promising step toward a commercial GMP manufacturing method to expand NK cells for therapeutic use.
Keywords: Cloudz microspheres; G-Rex6M; feeder-cell-free; human platelet lysate (hPL); natural killer (NK) cell; xeno-free.
Copyright © 2022 Johnson, Zale, Frary and Lomakin.
Conflict of interest statement
All authors are employees at Bio-Techne.
Figures
Similar articles
-
Evaluation of serum-free media formulations in feeder cell-stimulated expansion of natural killer cells.Cytotherapy. 2020 Jun;22(6):322-328. doi: 10.1016/j.jcyt.2020.02.002. Epub 2020 Apr 8. Cytotherapy. 2020. PMID: 32278551
-
Artificial feeder cells expressing ligands for killer cell immunoglobulin-like receptors and CD94/NKG2A for expansion of functional primary natural killer cells with tolerance to self.Cytotherapy. 2020 Jul;22(7):354-368. doi: 10.1016/j.jcyt.2020.02.004. Epub 2020 May 23. Cytotherapy. 2020. PMID: 32451262
-
Optimization of Human NK Cell Manufacturing: Fully Automated Separation, Improved Ex Vivo Expansion Using IL-21 with Autologous Feeder Cells, and Generation of Anti-CD123-CAR-Expressing Effector Cells.Hum Gene Ther. 2017 Oct;28(10):897-913. doi: 10.1089/hum.2017.157. Epub 2017 Aug 15. Hum Gene Ther. 2017. PMID: 28810809
-
Advances in NK cell production.Cell Mol Immunol. 2022 Apr;19(4):460-481. doi: 10.1038/s41423-021-00808-3. Epub 2022 Jan 5. Cell Mol Immunol. 2022. PMID: 34983953 Free PMC article. Review.
-
Selection and expansion of natural killer cells for NK cell-based immunotherapy.Cancer Immunol Immunother. 2016 Apr;65(4):477-84. doi: 10.1007/s00262-016-1792-y. Epub 2016 Jan 25. Cancer Immunol Immunother. 2016. PMID: 26810567 Free PMC article. Review.
Cited by
-
A Critical Role of Culture Medium Selection in Maximizing the Purity and Expansion of Natural Killer Cells.Cells. 2024 Jul 5;13(13):1148. doi: 10.3390/cells13131148. Cells. 2024. PMID: 38994999 Free PMC article.
-
Scalable process development of NK and CAR-NK expansion in a closed bioreactor.Front Immunol. 2024 Jul 24;15:1412378. doi: 10.3389/fimmu.2024.1412378. eCollection 2024. Front Immunol. 2024. PMID: 39114666 Free PMC article.
-
Beyond CAR-T: The rise of CAR-NK cell therapy in asthma immunotherapy.J Transl Med. 2024 Aug 5;22(1):736. doi: 10.1186/s12967-024-05534-8. J Transl Med. 2024. PMID: 39103889 Free PMC article. Review.
-
Promising approach for targeting ROBO1 with CAR NK cells to combat ovarian cancer primary tumor cells and organoids.Future Sci OA. 2024 Jul 11;10(1):2340186. doi: 10.2144/fsoa-2023-0135. Epub 2024 Jul 29. Future Sci OA. 2024. PMID: 39069888 Free PMC article.
-
Process engineering of natural killer cell-based immunotherapy.Trends Biotechnol. 2023 Oct;41(10):1314-1326. doi: 10.1016/j.tibtech.2023.03.018. Epub 2023 May 2. Trends Biotechnol. 2023. PMID: 37142447 Free PMC article. Review.
References
-
- Shaffer BC, Le Luduec J-B, Forlenza C, Jakubowski AA, Perales M-A, Young JW, et al. . Phase II Study of Haploidentical Natural Killer Cell Infusion for Treatment of Relapsed or Persistent Myeloid Malignancies Following Allogeneic Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant (2016) 22:705–9. doi: 10.1016/j.bbmt.2015.12.028 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

