The Pathophysiology of The Antiphospholipid Syndrome: A Perspective From The Blood Coagulation System
- PMID: 35317658
- PMCID: PMC8950029
- DOI: 10.1177/10760296221088576
The Pathophysiology of The Antiphospholipid Syndrome: A Perspective From The Blood Coagulation System
Abstract
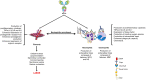
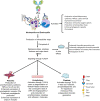
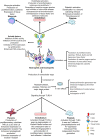
The antiphospholipid syndrome (APS), a systemic autoimmune disease characterized by a hypercoagulability associated to vascular thrombosis and/or obstetric morbidity, is caused by the presence of antiphospholipid antibodies such as lupus anticoagulant, anti-β-2-glycoprotein 1, and/or anticardiolipin antibodies. In the obstetrical APS, antiphospholipid antibodies induce the production of proinflammatory cytokines and tissue factor by placental tissues and recruited neutrophils. Moreover, antiphospholipid antibodies activate the complement system which, in turn, induces a positive feedback leading to recruitment of neutrophils as well as activation of the placenta. Activation of these cells triggers myometrial contractions and cervical ripening provoking the induction of labor. In thrombotic and obstetrical APS, antiphospholipid antibodies activate endothelial cells, platelets, and neutrophils and they may alter the multimeric pattern and concentration of von Willebrand factor, increase the concentration of thrombospondin 1, reduce the inactivation of factor XI by antithrombin, increase the activation of factor XII, and reduce the activity of tissue plasminogen activator with the subsequent production of plasmin. All these effects result in less permeable clots, denser, thinner, and with more branched fibrin fibers which are more difficult to lysate. As a consequence, thrombosis, the defining clinical criterion of APS, complicates the clinical course of the patient.
Keywords: antiphospholipid syndrome; autoantibodies; autoimmunity; miscarriage; thrombosis.
Conflict of interest statement
Figures
Similar articles
-
The role of thrombospondin-1 in the pathogenesis of antiphospholipid syndrome.J Autoimmun. 2020 Dec;115:102527. doi: 10.1016/j.jaut.2020.102527. Epub 2020 Jul 21. J Autoimmun. 2020. PMID: 32709480
-
Is There an Additional Value in Detecting Anticardiolipin and Anti-β2 glycoprotein I IgA Antibodies in the Antiphospholipid Syndrome?Thromb Haemost. 2020 Nov;120(11):1557-1568. doi: 10.1055/s-0040-1714653. Epub 2020 Jul 21. Thromb Haemost. 2020. PMID: 32696448
-
Antiphospholipid and antioangiogenic activity in females with recurrent miscarriage and antiphospholipid syndrome.Ann Clin Biochem. 2017 Sep;54(5):577-583. doi: 10.1177/0004563216672248. Epub 2016 Sep 16. Ann Clin Biochem. 2017. PMID: 27638930
-
Mechanisms of immunothrombosis and vasculopathy in antiphospholipid syndrome.Semin Immunopathol. 2022 May;44(3):347-362. doi: 10.1007/s00281-022-00916-w. Epub 2022 Feb 4. Semin Immunopathol. 2022. PMID: 35122116 Free PMC article. Review.
-
Pathophysiology of thrombosis and pregnancy morbidity in the antiphospholipid syndrome.Eur J Clin Invest. 2012 Oct;42(10):1126-35. doi: 10.1111/j.1365-2362.2012.02697.x. Epub 2012 Jul 12. Eur J Clin Invest. 2012. PMID: 22784367 Review.
Cited by
-
Antiphospholipid Antibody Testing in a Maximum Care Hospital: Method-Dependent Differences.J Clin Med. 2024 Aug 2;13(15):4528. doi: 10.3390/jcm13154528. J Clin Med. 2024. PMID: 39124794 Free PMC article.
-
Antiphospholipid Syndrome: Insights into Molecular Mechanisms and Clinical Manifestations.J Clin Med. 2024 Jul 18;13(14):4191. doi: 10.3390/jcm13144191. J Clin Med. 2024. PMID: 39064231 Free PMC article. Review.
-
Application of the thrombin generation assay in patients with antiphospholipid syndrome: A systematic review of the literature.Front Cardiovasc Med. 2023 Mar 28;10:1075121. doi: 10.3389/fcvm.2023.1075121. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37057100 Free PMC article. Review.
-
Obstetric antiphospholipid syndrome carries an increased lifetime risk for obstetric and thrombotic complications-a population-based study.Res Pract Thromb Haemost. 2024 Apr 29;8(4):102430. doi: 10.1016/j.rpth.2024.102430. eCollection 2024 May. Res Pract Thromb Haemost. 2024. PMID: 38798792 Free PMC article.
-
A Comparative Analysis of Anticardiolipin, Anti-Β2-Glycoprotein-1, and Lupus Anticoagulants in Saudi Women with Recurrent Spontaneous Abortions.J Pers Med. 2022 Dec 20;13(1):2. doi: 10.3390/jpm13010002. J Pers Med. 2022. PMID: 36675663 Free PMC article.
References
-
- Levy RA, Gómez-Puerta JA, Cervera R. History, classification, and subsets of the antiphospholipid syndrome. In: Cervera R, Espinosa G, Khamashta M, eds. Antiphospholipid Syndrome in Systemic Autoimmune Diseases. 2nd ed. Elsevier; 2017:1‐16.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

