Temperature impacts the environmental suitability for malaria transmission by Anopheles gambiae and Anopheles stephensi
- PMID: 35315521
- PMCID: PMC9357211
- DOI: 10.1002/ecy.3685
Temperature impacts the environmental suitability for malaria transmission by Anopheles gambiae and Anopheles stephensi
Abstract
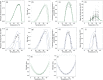
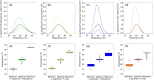
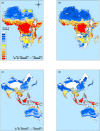
Extrinsic environmental factors influence the spatiotemporal dynamics of many organisms, including insects that transmit the pathogens responsible for vector-borne diseases (VBDs). Temperature is an especially important constraint on the fitness of a wide variety of ectothermic insects. A mechanistic understanding of how temperature impacts traits of ectotherms, and thus the distribution of ectotherms and vector-borne infections, is key to predicting the consequences of climate change on transmission of VBDs like malaria. However, the response of transmission to temperature and other drivers is complex, as thermal traits of ectotherms are typically nonlinear, and they interact to determine transmission constraints. In this study, we assess and compare the effect of temperature on the transmission of two malaria parasites, Plasmodium falciparum and Plasmodium vivax, by two malaria vector species, Anopheles gambiae and Anopheles stephensi. We model the nonlinear responses of temperature dependent mosquito and parasite traits (mosquito development rate, bite rate, fecundity, proportion of eggs surviving to adulthood, vector competence, mortality rate, and parasite development rate) and incorporate these traits into a suitability metric based on a model for the basic reproductive number across temperatures. Our model predicts that the optimum temperature for transmission suitability is similar for the four mosquito-parasite combinations assessed in this study, but may differ at the thermal limits. More specifically, we found significant differences in the upper thermal limit between parasites spread by the same mosquito (A. stephensi) and between mosquitoes carrying P. falciparum. In contrast, at the lower thermal limit the significant differences were primarily between the mosquito species that both carried the same pathogen (e.g., A. stephensi and A. gambiae both with P. falciparum). Using prevalence data, we show that the transmission suitability metric calculated from our mechanistic model is consistent with observed P. falciparum prevalence in Africa and Asia but is equivocal for P. vivax prevalence in Asia, and inconsistent with P. vivax prevalence in Africa. We mapped risk to illustrate the number of months various areas in Africa and Asia predicted to be suitable for malaria transmission based on this suitability metric. This mapping provides spatially explicit predictions for suitability and transmission risk.
Keywords: Africa; Asia; Plasmodium falciparum; Plasmodium vivax; basic reproductive number; malaria; mosquito life history; thermal performance curve; vector-borne diseases.
© 2022 The Authors. Ecology published by Wiley Periodicals LLC on behalf of The Ecological Society of America.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Mapping current and future thermal limits to suitability for malaria transmission by the invasive mosquito Anopheles stephensi.Malar J. 2023 Mar 21;22(1):104. doi: 10.1186/s12936-023-04531-4. Malar J. 2023. PMID: 36945014 Free PMC article.
-
Five years following first detection of Anopheles stephensi (Diptera: Culicidae) in Djibouti, Horn of Africa: populations established-malaria emerging.Parasitol Res. 2019 Mar;118(3):725-732. doi: 10.1007/s00436-019-06213-0. Epub 2019 Jan 22. Parasitol Res. 2019. PMID: 30671729
-
Age influences the thermal suitability of Plasmodium falciparum transmission in the Asian malaria vector Anopheles stephensi.Proc Biol Sci. 2020 Jul 29;287(1931):20201093. doi: 10.1098/rspb.2020.1093. Epub 2020 Jul 22. Proc Biol Sci. 2020. PMID: 32693720 Free PMC article.
-
Mathematical modeling of climate change and malaria transmission dynamics: a historical review.J Math Biol. 2018 Oct;77(4):857-933. doi: 10.1007/s00285-018-1229-7. Epub 2018 Apr 24. J Math Biol. 2018. PMID: 29691632 Review.
-
Systematic review of sporozoite infection rate of Anopheles mosquitoes in Ethiopia, 2001-2021.Parasit Vectors. 2023 Nov 27;16(1):437. doi: 10.1186/s13071-023-06054-y. Parasit Vectors. 2023. PMID: 38008761 Free PMC article. Review.
Cited by
-
Mean daily temperatures can predict the thermal limits of malaria transmission better than rate summation.bioRxiv [Preprint]. 2024 Sep 23:2024.09.20.614098. doi: 10.1101/2024.09.20.614098. bioRxiv. 2024. PMID: 39386442 Free PMC article. Preprint.
-
Influence of future climate scenarios using CMIP 5 data on malaria transmission in India.Malar J. 2024 Oct 9;23(1):301. doi: 10.1186/s12936-024-05129-0. Malar J. 2024. PMID: 39385165 Free PMC article.
-
Feeding habits and malaria parasite infection of Anopheles mosquitoes in selected agroecological areas of Northwestern Ethiopia.Parasit Vectors. 2024 Oct 3;17(1):412. doi: 10.1186/s13071-024-06496-y. Parasit Vectors. 2024. PMID: 39363366 Free PMC article.
-
Temperature dependence of mosquitoes: Comparing mechanistic and machine learning approaches.PLoS Negl Trop Dis. 2024 Sep 16;18(9):e0012488. doi: 10.1371/journal.pntd.0012488. eCollection 2024 Sep. PLoS Negl Trop Dis. 2024. PMID: 39283940 Free PMC article.
-
Warmer environmental temperature accelerates aging in mosquitoes, decreasing longevity and worsening infection outcomes.Immun Ageing. 2024 Sep 11;21(1):61. doi: 10.1186/s12979-024-00465-w. Immun Ageing. 2024. PMID: 39261928 Free PMC article.
References
-
- Abram, P. K. , Boivin G., Moiroux J., and Brodeur J.. 2017. “Behavioural Effects of Temperature on Ectothermic Animals: Unifying Thermal Physiology and Behavioural Plasticity.” Biological Reviews 92: 1859–76. - PubMed
-
- Aho, K. , Derryberry D., and Peterson T.. 2014. “Model Selection for Ecologists: The Worldviews of AIC and BIC.” Ecology 95: 631–6. - PubMed
-
- Amarasekare, P. , and Savage V.. 2012. “A Framework for Elucidating the Temperature Dependence of Fitness.” The American Naturalist 179: 178–91. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
