Stiffness- and Bioactive Factor-Mediated Protection of Self-Assembled Cartilage against Macrophage Challenge in a Novel Co-Culture System
- PMID: 35313741
- PMCID: PMC9137312
- DOI: 10.1177/19476035221081466
Stiffness- and Bioactive Factor-Mediated Protection of Self-Assembled Cartilage against Macrophage Challenge in a Novel Co-Culture System
Abstract
Objective: Tissue-engineered cartilage implants must withstand the potential inflammatory and joint loading environment for successful long-term repair of defects. The work's objectives were to develop a novel, direct cartilage-macrophage co-culture system and to characterize interactions between self-assembled neocartilage and differentially stimulated macrophages.
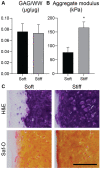
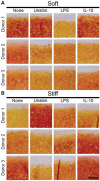
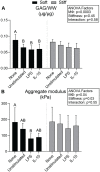
Design: In study 1, it was hypothesized that the proinflammatory response of macrophages would intensify with increasing construct stiffness; it was expected that the neocartilage would display a decrease in mechanical properties after co-culture. In study 2, it was hypothesized that bioactive factors would protect neocartilage properties during macrophage co-culture. Also, it was hypothesized that interleukin 10 (IL-10)-stimulated macrophages would improve neocartilage mechanical properties compared to lipopolysaccharide (LPS)-stimulated macrophages.
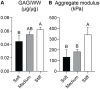
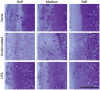
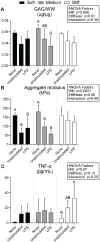
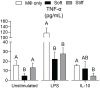
Results: As hypothesized, stiffer neocartilage elicited a heightened proinflammatory macrophage response, increasing tumor necrosis factor alpha (TNF-α) secretion by 5.47 times when LPS-stimulated compared to construct-only controls. Interestingly, this response did not adversely affect construct properties for the stiffest neocartilage but did correspond to a significant decrease in aggregate modulus for soft and medium stiffness constructs. In addition, bioactive factor-treated constructs were protected from macrophage challenge compared to chondrogenic medium-treated constructs, but IL-10 did not improve neocartilage properties, although stiff constructs appeared to bolster the anti-inflammatory nature of IL-10-stimulated macrophages. However, co-culture of bioactive factor-treated constructs with LPS-treated macrophages reduced TNF-α secretion by over 4 times compared to macrophage-only controls.
Conclusions: In conclusion, neocartilage stiffness can mediate macrophage behavior, but stiffness and bioactive factors prevent macrophage-induced degradation. Ultimately, this co-culture system could be utilized for additional studies to develop the burgeoning field of cartilage mechano-immunology.
Keywords: cartilage; immunology; macrophage; tissue engineering.
Conflict of interest statement
Figures
Similar articles
-
Effects of passage number and post-expansion aggregate culture on tissue engineered, self-assembled neocartilage.Acta Biomater. 2016 Oct 1;43:150-159. doi: 10.1016/j.actbio.2016.07.044. Epub 2016 Jul 28. Acta Biomater. 2016. PMID: 27475530 Free PMC article.
-
The Effect of Neonatal, Juvenile, and Adult Donors on Rejuvenated Neocartilage Functional Properties.Tissue Eng Part A. 2022 May;28(9-10):383-393. doi: 10.1089/ten.TEA.2021.0167. Epub 2022 Jan 21. Tissue Eng Part A. 2022. PMID: 34605665 Free PMC article.
-
The functionality and translatability of neocartilage constructs are improved with the combination of fluid-induced shear stress and bioactive factors.FASEB J. 2022 Apr;36(4):e22225. doi: 10.1096/fj.202101699R. FASEB J. 2022. PMID: 35224777 Free PMC article.
-
Engineering biomechanically functional neocartilage derived from expanded articular chondrocytes through the manipulation of cell-seeding density and dexamethasone concentration.J Tissue Eng Regen Med. 2017 Aug;11(8):2323-2332. doi: 10.1002/term.2132. Epub 2016 May 3. J Tissue Eng Regen Med. 2017. PMID: 27138113
-
In Vitro Effects of Bupivacaine on the Viability and Mechanics of Native and Engineered Cartilage Grafts.Am J Sports Med. 2021 Apr;49(5):1305-1312. doi: 10.1177/0363546521995184. Epub 2021 Mar 5. Am J Sports Med. 2021. PMID: 33667144
Cited by
-
Recent advancements in cartilage tissue engineering innovation and translation.Nat Rev Rheumatol. 2024 Jun;20(6):323-346. doi: 10.1038/s41584-024-01118-4. Epub 2024 May 13. Nat Rev Rheumatol. 2024. PMID: 38740860 Review.
-
Engineering Cell-ECM-Material Interactions for Musculoskeletal Regeneration.Bioengineering (Basel). 2023 Apr 7;10(4):453. doi: 10.3390/bioengineering10040453. Bioengineering (Basel). 2023. PMID: 37106640 Free PMC article. Review.
-
Nanotopographical cues for regulation of macrophages and osteoclasts: emerging opportunities for osseointegration.J Nanobiotechnology. 2022 Dec 3;20(1):510. doi: 10.1186/s12951-022-01721-1. J Nanobiotechnology. 2022. PMID: 36463225 Free PMC article. Review.
-
Astaxanthin mediated repair of tBHP-Induced cellular injury in chondrocytes.Redox Rep. 2024 Dec;29(1):2422271. doi: 10.1080/13510002.2024.2422271. Epub 2024 Nov 4. Redox Rep. 2024. PMID: 39495906 Free PMC article.
-
Proteomic, mechanical, and biochemical development of tissue-engineered neocartilage.Biomater Res. 2022 Jul 22;26(1):34. doi: 10.1186/s40824-022-00284-4. Biomater Res. 2022. PMID: 35869489 Free PMC article.

