The Role of Peroxisome Proliferator-Activated Receptor Gamma and Atherosclerosis: Post-translational Modification and Selective Modulators
- PMID: 35309069
- PMCID: PMC8924581
- DOI: 10.3389/fphys.2022.826811
The Role of Peroxisome Proliferator-Activated Receptor Gamma and Atherosclerosis: Post-translational Modification and Selective Modulators
Abstract
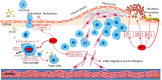
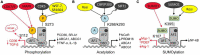
Atherosclerosis is the hallmark of cardiovascular disease (CVD) which is a leading cause of death in type 2 diabetes patients, and glycemic control is not beneficial in reducing the potential risk of CVD. Clinically, it was shown that Thiazolidinediones (TZDs), a class of peroxisome proliferator-activated receptor gamma (PPARγ) agonists, are insulin sensitizers with reducing risk of CVD, while the potential adverse effects, such as weight gain, fluid retention, bone loss, and cardiovascular risk, restricts its use in diabetic treatment. PPARγ, a ligand-activated nuclear receptor, has shown to play a crucial role in anti-atherosclerosis by promoting cholesterol efflux, repressing monocytes infiltrating into the vascular intima under endothelial layer, their transformation into macrophages, and inhibiting vascular smooth muscle cells proliferation as well as migration. The selective activation of subsets of PPARγ targets, such as through PPARγ post-translational modification, is thought to improve the safety profile of PPARγ agonists. Here, this review focuses on the significance of PPARγ activity regulation (selective activation and post-translational modification) in the occurrence, development and treatment of atherosclerosis, and further clarifies the value of PPARγ as a safe therapeutic target for anti-atherosclerosis especially in diabetic treatment.
Keywords: PPARγ; atherosclerosis; cardiovascular disease; post-translational modifications; selective modulators.
Copyright © 2022 Yin, Wang, Shi, Ji and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Potent Anti-Diabetic Actions of a Novel Non-Agonist PPARγ Ligand that Blocks Cdk5-Mediated Phosphorylation.2011 Jul 5 [updated 2013 Mar 7]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010–. 2011 Jul 5 [updated 2013 Mar 7]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010–. PMID: 23762958 Free Books & Documents. Review.
-
PPARγ signaling and emerging opportunities for improved therapeutics.Pharmacol Res. 2016 Sep;111:76-85. doi: 10.1016/j.phrs.2016.02.028. Epub 2016 Jun 4. Pharmacol Res. 2016. PMID: 27268145 Free PMC article. Review.
-
[Effects of PPARgamma agonist on dyslipidemia and atherosclerosis].Nihon Rinsho. 2010 Feb;68(2):294-8. Nihon Rinsho. 2010. PMID: 20158099 Review. Japanese.
-
Role of peroxisome proliferator-activated receptor-γ in atherosclerosis: an update.Circ J. 2011;75(3):528-35. doi: 10.1253/circj.cj-11-0060. Epub 2011 Feb 11. Circ J. 2011. PMID: 21325726 Review.
-
The role of peroxisome proliferator-activated receptor γ in lipid metabolism and inflammation in atherosclerosis.Cell Biol Int. 2023 Sep;47(9):1469-1487. doi: 10.1002/cbin.12065. Epub 2023 Jun 27. Cell Biol Int. 2023. PMID: 37369936 Review.
Cited by
-
PPARγ in Atherosclerotic Endothelial Dysfunction: Regulatory Compounds and PTMs.Int J Mol Sci. 2023 Sep 24;24(19):14494. doi: 10.3390/ijms241914494. Int J Mol Sci. 2023. PMID: 37833942 Free PMC article. Review.
-
High plasma concentrations of vanin-1 in patients with coronary artery disease.Heart Vessels. 2024 Jan;39(1):10-17. doi: 10.1007/s00380-023-02305-1. Epub 2023 Aug 15. Heart Vessels. 2024. PMID: 37582951
-
[Pharmacodynamics of Qingxin Jieyu Granules for treatment of atherosclerosis and its regulatory mechanism for lipid metabolism].Nan Fang Yi Ke Da Xue Xue Bao. 2024 Aug 20;44(8):1518-1528. doi: 10.12122/j.issn.1673-4254.2024.08.10. Nan Fang Yi Ke Da Xue Xue Bao. 2024. PMID: 39276047 Free PMC article. Chinese.
-
Molecular mechanism of Cuscutae semen-radix rehmanniae praeparata in relieving reproductive injury of male rats induced with tripterygium wilfordii multiglycosides: A tandem mass tag-based proteomics analysis.Front Pharmacol. 2023 Feb 17;14:1050907. doi: 10.3389/fphar.2023.1050907. eCollection 2023. Front Pharmacol. 2023. PMID: 36874004 Free PMC article.
-
Lobeglitazone, a novel thiazolidinedione, for secondary prevention in patients with ischemic stroke: a nationwide nested case-control study.Cardiovasc Diabetol. 2023 May 5;22(1):106. doi: 10.1186/s12933-023-01841-4. Cardiovasc Diabetol. 2023. PMID: 37147722 Free PMC article.
References
-
- Akiyama T. E., Sakai S., Lambert G., Nicol C. J., Matsusue K., Pimprale S., et al. (2002). Conditional disruption of the peroxisome proliferator-activated receptor gamma gene in mice results in lowered expression of ABCA1, ABCG1, and apoE in macrophages and reduced cholesterol efflux. Mol. Cell. Biol. 22 2607–2619. 10.1128/MCB.22.8.2607-2619.2002 - DOI - PMC - PubMed
-
- Babaev V. R., Yancey P. G., Ryzhov S. V., Kon V., Breyer M. D., Magnuson M. A., et al. (2005). Conditional knockout of macrophage PPARgamma increases atherosclerosis in C57BL/6 and low-density lipoprotein receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 25 1647–1653. 10.1161/01.ATV.0000173413.31789.1a - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

