Aducanumab Therapy to Treat Alzheimer's Disease: A Narrative Review
- PMID: 35308835
- PMCID: PMC8926483
- DOI: 10.1155/2022/9343514
Aducanumab Therapy to Treat Alzheimer's Disease: A Narrative Review
Abstract
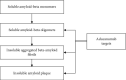
Background: Aducanumab, a new monoclonal antibody that targets β-amyloid aggregates, has been granted conditional approval by the U.S. FDA for treatment of mild Alzheimer's disease (AD). The approval of this drug without a confirmed significant clinical impact has resulted in several debates.
Objective: In this narrative review, aducanumab approval-related controversy, the drug's pharmacokinetics and pharmacodynamic characteristics, evidence from the efficacy and safety trials of aducanumab, implications of the drug approval, and the future directions in the management of patients with AD are summarized.
Methods: Using relevant keywords, Google Scholar, Web of Science, and MEDLINE databases and manufacturer's website were searched.
Results: Infusion of aducanumab at a higher dose resulted in a modest slowing of cognitive decline among patients with mild cognitive impairment or early-onset AD dementia. The drug however can cause amyloid-related imaging abnormalities. Due to modest impact on cognition, the use of this drug by patients with AD will most likely be limited. The manufacturer is required to run an extended phase IIIb trial to verify the benefit of this drug. Access to therapy requires a careful selection of patients and periodic monitoring to ensure the optimal use of the drug.
Conclusion: Despite the limitations, aducanumab is the first disease-modifying therapy approved for the treatment of AD. Aducanumab addresses a part of the pathogenesis of AD; therefore, drugs that can act on multiple targets are needed. In addition, the search for preventive strategies, validated plasma-based assays, and newer drugs for AD, which are effective, safe, convenient, and affordable, is vital.
Copyright © 2022 Semira Abdi Beshir et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
Similar articles
-
Aducanumab for Alzheimer's Disease: Summarized Data From EMERGE, ENGAGE, and PRIME Studies.Sr Care Pharm. 2022 Aug 1;37(8):329-334. doi: 10.4140/TCP.n.2022.329. Sr Care Pharm. 2022. PMID: 35879846
-
Profiling aducanumab as a treatment option for Alzheimer's disease: an overview of efficacy, safety and tolerability.Expert Rev Neurother. 2024 Nov;24(11):1045-1053. doi: 10.1080/14737175.2024.2402058. Epub 2024 Sep 18. Expert Rev Neurother. 2024. PMID: 39291991 Review.
-
Aducanumab: evidence from clinical trial data and controversies.Drugs Context. 2021 Oct 4;10:2021-7-3. doi: 10.7573/dic.2021-7-3. eCollection 2021. Drugs Context. 2021. PMID: 34650610 Free PMC article.
-
Aducanumab, a Novel Anti-Amyloid Monoclonal Antibody, for the Treatment of Alzheimer's Disease: A Comprehensive Review.Health Psychol Res. 2022 Jan 30;10(1):31925. doi: 10.52965/001c.31925. eCollection 2022. Health Psychol Res. 2022. PMID: 35928986 Free PMC article.
-
A Primer on the Evolution of Aducanumab: The First Antibody Approved for Treatment of Alzheimer's Disease.J Alzheimers Dis. 2021;83(4):1537-1552. doi: 10.3233/JAD-215065. J Alzheimers Dis. 2021. PMID: 34366359 Review.
Cited by
-
Understanding neuropsychiatric symptoms in Alzheimer's disease: challenges and advances in diagnosis and treatment.Front Neurosci. 2023 Sep 5;17:1263771. doi: 10.3389/fnins.2023.1263771. eCollection 2023. Front Neurosci. 2023. PMID: 37732300 Free PMC article. Review.
-
Engineered Antibodies to Improve Efficacy against Neurodegenerative Disorders.Int J Mol Sci. 2024 Jun 18;25(12):6683. doi: 10.3390/ijms25126683. Int J Mol Sci. 2024. PMID: 38928395 Free PMC article. Review.
-
Multi-modal neuroprotection of Argemone mexicana L. against Alzheimer's disease: In vitro and in silico study.Heliyon. 2024 Aug 30;10(17):e37178. doi: 10.1016/j.heliyon.2024.e37178. eCollection 2024 Sep 15. Heliyon. 2024. PMID: 39286063 Free PMC article.
-
Advances in Therapeutics to Alleviate Cognitive Decline and Neuropsychiatric Symptoms of Alzheimer's Disease.Int J Mol Sci. 2024 May 9;25(10):5169. doi: 10.3390/ijms25105169. Int J Mol Sci. 2024. PMID: 38791206 Free PMC article. Review.
-
Functional near-infrared spectroscopy and vagus somatosensory evoked potentials add to the power of established parameters such as poor cognitive performance, dsyosmia and APOe genotype to predict cognitive decline over 8 years in the elderly.J Neural Transm (Vienna). 2024 Nov 13. doi: 10.1007/s00702-024-02859-y. Online ahead of print. J Neural Transm (Vienna). 2024. PMID: 39535568
References
-
- World Health Organization. Dementia . 2021. https://www.who.int/news-room/fact-sheets/detail/dementia .
-
- Dementia statistics. Alzheimer’s Disease International . 2021 https://www.alzint.org/about/dementia-facts-figures/dementia-statistics/
-
- Plascencia-Villa G., Perry G. Alzheimer’s disease pharmacology. Reference Module in Biomedical Sciences . 2021 doi: 10.1101/2021.12.03.21267235. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources