The innate immune system stimulating cytokine GM-CSF improves learning/memory and interneuron and astrocyte brain pathology in Dp16 Down syndrome mice and improves learning/memory in wild-type mice
- PMID: 35307513
- PMCID: PMC9045510
- DOI: 10.1016/j.nbd.2022.105694
The innate immune system stimulating cytokine GM-CSF improves learning/memory and interneuron and astrocyte brain pathology in Dp16 Down syndrome mice and improves learning/memory in wild-type mice
Abstract
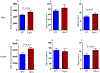
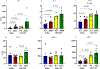
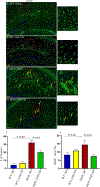
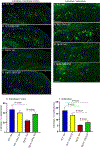
Down syndrome (DS) is characterized by chronic neuroinflammation, peripheral inflammation, astrogliosis, imbalanced excitatory/inhibitory neuronal function, and cognitive deficits in both humans and mouse models. Suppression of inflammation has been proposed as a therapeutic approach to treating DS co-morbidities, including intellectual disability (DS/ID). Conversely, we discovered previously that treatment with the innate immune system stimulating cytokine granulocyte-macrophage colony-stimulating factor (GM-CSF), which has both pro- and anti-inflammatory activities, improved cognition and reduced brain pathology in a mouse model of Alzheimer's disease (AD), another inflammatory disorder, and improved cognition and reduced biomarkers of brain pathology in a phase II trial of humans with mild-to-moderate AD. To investigate the effects of GM-CSF treatment on DS/ID in the absence of AD, we assessed behavior and brain pathology in 12-14 month-old DS mice (Dp[16]1Yey) and their wild-type (WT) littermates, neither of which develop amyloid, and found that subcutaneous GM-CSF treatment (5 μg/day, five days/week, for five weeks) improved performance in the radial arm water maze in both Dp16 and WT mice compared to placebo. Dp16 mice also showed abnormal astrocyte morphology, increased percent area of GFAP staining in the hippocampus, clustering of astrocytes in the hippocampus, and reduced numbers of calretinin-positive interneurons in the entorhinal cortex and subiculum, and all of these brain pathologies were improved by GM-CSF treatment. These findings suggest that stimulating and/or modulating inflammation and the innate immune system with GM-CSF treatment may enhance cognition in both people with DS/ID and in the typical aging population.
Keywords: Alzheimer's disease (AD); Astrocyte; Calretinin; Cognition; Down syndrome (DS); Dp(16)1Yey (Dp16); Glial-fibrillary acidic protein (GFAP); Granulocyte-macrophage colony-stimulating factor (GM-CSF); Intellectual disability (ID); Interneuron.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests
The authors declare no competing interests. Dr. Boyd was recently hired by Partner Therapeutics, the manufacturer of human recombinant GM-CSF.
Figures



Similar articles
-
Granulocyte colony stimulating factor decreases brain amyloid burden and reverses cognitive impairment in Alzheimer's mice.Neuroscience. 2009 Sep 29;163(1):55-72. doi: 10.1016/j.neuroscience.2009.05.071. Epub 2009 Jun 14. Neuroscience. 2009. PMID: 19500657 Free PMC article.
-
GM-CSF upregulated in rheumatoid arthritis reverses cognitive impairment and amyloidosis in Alzheimer mice.J Alzheimers Dis. 2010;21(2):507-18. doi: 10.3233/JAD-2010-091471. J Alzheimers Dis. 2010. PMID: 20555144 Free PMC article.
-
Astroglial Activation Is Exacerbated in a Down Syndrome Mouse Model.Neuroscience. 2024 May 24;547:88-97. doi: 10.1016/j.neuroscience.2024.04.003. Epub 2024 Apr 12. Neuroscience. 2024. PMID: 38615829
-
Innate Immune System Activation and Neuroinflammation in Down Syndrome and Neurodegeneration: Therapeutic Targets or Partners?Front Aging Neurosci. 2021 Sep 16;13:718426. doi: 10.3389/fnagi.2021.718426. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34603007 Free PMC article. Review.
-
The Role of Granulocyte-Macrophage Colony-Stimulating Factor in Murine Models of Multiple Sclerosis.Cells. 2020 Mar 4;9(3):611. doi: 10.3390/cells9030611. Cells. 2020. PMID: 32143326 Free PMC article. Review.
Cited by
-
Rodent Modeling of Alzheimer's Disease in Down Syndrome: In vivo and ex vivo Approaches.Front Neurosci. 2022 Jun 7;16:909669. doi: 10.3389/fnins.2022.909669. eCollection 2022. Front Neurosci. 2022. PMID: 35747206 Free PMC article. Review.
-
Effects of Major Royal Jelly Proteins on the Immune Response and Gut Microbiota Composition in Cyclophosphamide-Treated Mice.Nutrients. 2023 Feb 15;15(4):974. doi: 10.3390/nu15040974. Nutrients. 2023. PMID: 36839331 Free PMC article.
-
Treatment with Granulocyte-Macrophage Colony-Stimulating Factor Reduces Viral Titers in the Brains of West Nile Virus-Infected Mice and Improves Survival.J Virol. 2023 Mar 30;97(3):e0180522. doi: 10.1128/jvi.01805-22. Epub 2023 Feb 21. J Virol. 2023. PMID: 36802227 Free PMC article.
-
Editorial: Physiology and pathology of neuroglia.Front Cell Neurosci. 2023 Jul 18;17:1246885. doi: 10.3389/fncel.2023.1246885. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37534041 Free PMC article. No abstract available.
-
Prenatal treatment with preimplantation factor improves early postnatal neurogenesis and cognitive impairments in a mouse model of Down syndrome.Cell Mol Life Sci. 2024 May 13;81(1):215. doi: 10.1007/s00018-024-05245-9. Cell Mol Life Sci. 2024. PMID: 38739166 Free PMC article.
References
-
- Opitz JM, Gilbert-Barness EF. Reflections on the pathogenesis of Down syndrome. Am J Med Genet Suppl 1990;7:38–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

