Acute and Delayed Effects of Stress Eliciting Post-Traumatic Stress-Like Disorder Differentially Alters Fecal Microbiota Composition in a Male Mouse Model
- PMID: 35300376
- PMCID: PMC8921487
- DOI: 10.3389/fcimb.2022.810815
Acute and Delayed Effects of Stress Eliciting Post-Traumatic Stress-Like Disorder Differentially Alters Fecal Microbiota Composition in a Male Mouse Model
Abstract
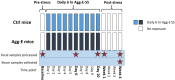
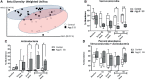
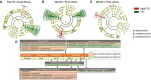
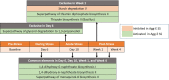
The association between the shift in fecal resident microbiome and social conflicts with long-term consequences on psychological plasticity, such as the development of post-traumatic stress disorder (PTSD), is yet to be comprehended. We developed an aggressor-exposed (Agg-E) social stress (SS) mouse model to mimic warzone-like conflicts, where random life-threatening interactions took place between naïve intruder mice and aggressive resident mice. Gradually these Agg-E mice developed distinct characteristics simulating PTSD-like aspects, whereas the control mice not exposed to Agg-E SS demonstrated distinct phenotypes. To further investigate the role of Agg-E SS on the resident microbiome, 16S rRNA gene sequencing was assayed using fecal samples collected at pre-, during, and post-SS time points. A time agonist shift in the fecal microbial composition of Agg-E mice in contrast to its controls suggested a persistent impact of Agg-E SS on resident microbiota. At the taxonomic level, Agg-E SS caused a significant shift in the time-resolved ratios of Firmicutes and Bacteroidetes abundance. Furthermore, Agg-E SS caused diverging shifts in the relative abundances of Verrucomicrobia and Actinobacteria. An in silico estimation of genomic potential identified a potentially perturbed cluster of bioenergetic networks, which became increasingly enriched with time since the termination of Agg-E SS. Supported by a growing number of studies, our results indicated the roles of the microbiome in a wide range of phenotypes that could mimic the comorbidities of PTSD, which would be directly influenced by energy deficiency. Together, the present work suggested the fecal microbiome as a potential tool to manage long-term effects of social conflicts, including the management of PTSD.
Keywords: C57BL/6J; PTSD; microbiome; social defeat; stress.
Copyright © 2022 Hoke, Chakraborty, Gautam, Hammamieh and Jett.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Altered fecal microbiota composition in all male aggressor-exposed rodent model simulating features of post-traumatic stress disorder.J Neurosci Res. 2018 Jul;96(7):1311-1323. doi: 10.1002/jnr.24229. Epub 2018 Apr 6. J Neurosci Res. 2018. PMID: 29633335
-
Gene and stress history interplay in emergence of PTSD-like features.Behav Brain Res. 2015 Oct 1;292:266-77. doi: 10.1016/j.bbr.2015.05.038. Epub 2015 May 27. Behav Brain Res. 2015. PMID: 26025510
-
Brain transcriptome profiles in mouse model simulating features of post-traumatic stress disorder.Mol Brain. 2015 Feb 28;8:14. doi: 10.1186/s13041-015-0104-3. Mol Brain. 2015. PMID: 25888136 Free PMC article.
-
Genome to Phenome: A Systems Biology Approach to PTSD Using an Animal Model.Methods Mol Biol. 2017;1598:117-154. doi: 10.1007/978-1-4939-6952-4_6. Methods Mol Biol. 2017. PMID: 28508360 Review.
-
[Posttraumatic stress disorder (PTSD) as a consequence of the interaction between an individual genetic susceptibility, a traumatogenic event and a social context].Encephale. 2012 Oct;38(5):373-80. doi: 10.1016/j.encep.2011.12.003. Epub 2012 Jan 24. Encephale. 2012. PMID: 23062450 Review. French.
Cited by
-
Severe, short-term sleep restriction reduces gut microbiota community richness but does not alter intestinal permeability in healthy young men.Sci Rep. 2023 Jan 5;13(1):213. doi: 10.1038/s41598-023-27463-0. Sci Rep. 2023. PMID: 36604516 Free PMC article.
-
The emerging role of the gut microbiome in posttraumatic stress disorder.Brain Behav Immun. 2023 Nov;114:360-370. doi: 10.1016/j.bbi.2023.09.005. Epub 2023 Sep 7. Brain Behav Immun. 2023. PMID: 37689277 Free PMC article. Review.
-
Metabolites: a converging node of host and microbe to explain meta-organism.Front Microbiol. 2024 Mar 5;15:1337368. doi: 10.3389/fmicb.2024.1337368. eCollection 2024. Front Microbiol. 2024. PMID: 38505556 Free PMC article. Review.
-
Ionizing Radiation Dose Differentially Affects the Host-Microbe Relationship over Time.Microorganisms. 2024 Sep 30;12(10):1995. doi: 10.3390/microorganisms12101995. Microorganisms. 2024. PMID: 39458305 Free PMC article.
-
The Role of Gut Dysbiosis in the Pathophysiology of Neuropsychiatric Disorders.Cells. 2022 Dec 23;12(1):54. doi: 10.3390/cells12010054. Cells. 2022. PMID: 36611848 Free PMC article. Review.
References
-
- Aguayo FI, Pacheco A., García-Rojo G. J., Pizarro-Bauerle J. A., Doberti A. V., Tejos M., et al. . (2018). Matrix Metalloproteinase 9 Displays a Particular Time Response to Acute Stress: Variation in its Levels and Activity Distribution in Rat Hippocampus. ACS Chem. Neurosci. 9 (5), 945–956. doi: 10.1021/acschemneuro.7b00387 - DOI - PubMed
-
- Al-Sadi R., Youssef M., Rawat M., Guo S., Dokladny K., Haque M., et al. . (2019). MMP-9-Induced Increase in Intestinal Epithelial Tight Permeability is Mediated by P38 Kinase Signaling Pathway Activation of MLCK Gene. Am. J. Physiol. Gastrointest Liver Physiol. 316 (2), G278–G290. doi: 10.1152/ajpgi.00126.2018 - DOI - PMC - PubMed
-
- Anderson M. J. (2001). A New Method for non-Parametric Multivariate Analysis of Variance. Austral Ecol. 26, 32–46. doi: 10.1111/j.1442-9993.2001.01070.pp.x - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

