ApoE Cascade Hypothesis in the pathogenesis of Alzheimer's disease and related dementias
- PMID: 35298921
- PMCID: PMC9035117
- DOI: 10.1016/j.neuron.2022.03.004
ApoE Cascade Hypothesis in the pathogenesis of Alzheimer's disease and related dementias
Abstract
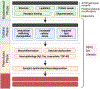
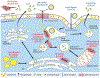
The ε4 allele of the apolipoprotein E gene (APOE4) is a strong genetic risk factor for Alzheimer's disease (AD) and several other neurodegenerative conditions, including Lewy body dementia (LBD). The three APOE alleles encode protein isoforms that differ from one another only at amino acid positions 112 and 158: apoE2 (C112, C158), apoE3 (C112, R158), and apoE4 (R112, R158). Despite progress, it remains unclear how these small amino acid differences in apoE sequence among the three isoforms lead to profound effects on aging and disease-related pathways. Here, we propose a novel "ApoE Cascade Hypothesis" in AD and age-related cognitive decline, which states that the biochemical and biophysical properties of apoE impact a cascade of events at the cellular and systems levels, ultimately impacting aging-related pathogenic conditions including AD. As such, apoE-targeted therapeutic interventions are predicted to be more effective by addressing the biochemical phase of the cascade.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests G.B. consults for SciNeuro and Lexeo, has consulted for Vida Ventures, AbbVie, E-Scape, and Eisai, is on the scientific advisory board of Kisbee Therapeutics, and serves as a Co-Editor-in-Chief for Molecular Neurodegeneration. D.M.H. is as an inventor on a patent licensed by Washington University to C2N Diagnostics on the therapeutic use of anti-tau antibodies. D.M.H. co-founded and is on the scientific advisory board of C2N Diagnostics. C2N Diagnostics has licensed certain anti-tau antibodies to AbbVie for therapeutic development. D.M.H. is on the scientific advisory board of Denali, Genentech, and Cajal Neurosciences and consults for Eli Lilly. D.M.H. receives sponsored research agreements to Washington University from NextCure, C2N Diagnostics, Yumanity, Eli Lilly, and Novartis. A.M.G. is on the scientific advisory board of Genentech and has consulted for Cognition Therapeutics and AbbVie. The other authors declare no competing interests.
Figures

Similar articles
-
Plasma ApoE4 Levels Are Lower than ApoE2 and ApoE3 Levels, and Not Associated with Plasma Aβ40/42 Ratio as a Biomarker of Amyloid-β Amyloidosis in Alzheimer's Disease.J Alzheimers Dis. 2023;93(1):333-348. doi: 10.3233/JAD-220996. J Alzheimers Dis. 2023. PMID: 36970894
-
Differential Signaling Mediated by ApoE2, ApoE3, and ApoE4 in Human Neurons Parallels Alzheimer's Disease Risk.J Neurosci. 2019 Sep 11;39(37):7408-7427. doi: 10.1523/JNEUROSCI.2994-18.2019. Epub 2019 Jul 22. J Neurosci. 2019. PMID: 31331998 Free PMC article.
-
Apolipoprotein E and oxidative stress in brain with relevance to Alzheimer's disease.Neurobiol Dis. 2020 May;138:104795. doi: 10.1016/j.nbd.2020.104795. Epub 2020 Feb 6. Neurobiol Dis. 2020. PMID: 32036033 Free PMC article. Review.
-
Opposing Effects of ApoE2 and ApoE4 on Glycolytic Metabolism in Neuronal Aging Supports a Warburg Neuroprotective Cascade against Alzheimer's Disease.Cells. 2023 Jan 25;12(3):410. doi: 10.3390/cells12030410. Cells. 2023. PMID: 36766752 Free PMC article.
-
Apolipoprotein E isoforms in Alzheimer's disease pathology and etiology.Microsc Res Tech. 2000 Aug 15;50(4):278-81. doi: 10.1002/1097-0029(20000815)50:4<278::AID-JEMT5>3.0.CO;2-T. Microsc Res Tech. 2000. PMID: 10936880 Review.
Cited by
-
Cell type-specific roles of APOE4 in Alzheimer disease.Nat Rev Neurosci. 2024 Feb;25(2):91-110. doi: 10.1038/s41583-023-00776-9. Epub 2024 Jan 8. Nat Rev Neurosci. 2024. PMID: 38191720 Free PMC article. Review.
-
The Brain-Gut Axis, an Important Player in Alzheimer and Parkinson Disease: A Narrative Review.J Clin Med. 2024 Jul 15;13(14):4130. doi: 10.3390/jcm13144130. J Clin Med. 2024. PMID: 39064171 Free PMC article. Review.
-
Lipid Trajectories Improve Risk Models for Alzheimer's Disease and Mild Cognitive Impairment.medRxiv [Preprint]. 2024 Sep 28:2024.09.27.24314494. doi: 10.1101/2024.09.27.24314494. medRxiv. 2024. Update in: J Lipid Res. 2024 Nov 23;66(1):100714. doi: 10.1016/j.jlr.2024.100714 PMID: 39399044 Free PMC article. Updated. Preprint.
-
Advancements in APOE and dementia research: Highlights from the 2023 AAIC Advancements: APOE conference.Alzheimers Dement. 2024 Sep;20(9):6590-6605. doi: 10.1002/alz.13877. Epub 2024 Jul 19. Alzheimers Dement. 2024. PMID: 39031528 Free PMC article. Review.
-
Effects of obesogenic diet and 17β-estradiol in female mice with APOE 3/3, 3/4, and 4/4 genotypes.Front Aging Neurosci. 2024 Sep 13;16:1415072. doi: 10.3389/fnagi.2024.1415072. eCollection 2024. Front Aging Neurosci. 2024. PMID: 39347015 Free PMC article.
References
-
- Arboleda-Velasquez JF, Lopera F, O'Hare M, Delgado-Tirado S, Marino C, Chmielewska N, Saez-Torres KL, Amarnani D, Schultz AP, Sperling RA, et al. (2019). Resistance to autosomal dominant Alzheimer's disease in an APOE3 Christchurch homozygote: a case report. Nat Med 25, 1680–1683. 10.1038/s41591-019-0611-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

