Dictyostelium spastin is involved in nuclear envelope dynamics during semi-closed mitosis
- PMID: 35298348
- PMCID: PMC8932920
- DOI: 10.1080/19491034.2022.2047289
Dictyostelium spastin is involved in nuclear envelope dynamics during semi-closed mitosis
Abstract
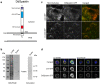
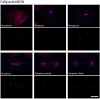
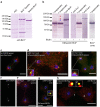
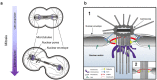
Dictyostelium amoebae perform a semi-closed mitosis, in which the nuclear envelope is fenestrated at the insertion sites of the mitotic centrosomes and around the central spindle during karyokinesis. During late telophase the centrosome relocates to the cytoplasmic side of the nucleus, the central spindle disassembles and the nuclear fenestrae become closed. Our data indicate that Dictyostelium spastin (DdSpastin) is a microtubule-binding and severing type I membrane protein that plays a role in this process. Its mitotic localization is in agreement with a requirement for the removal of microtubules that would hinder closure of the fenestrae. Furthermore, DdSpastin interacts with the HeH/ LEM-family protein Src1 in BioID analyses as well as the inner nuclear membrane protein Sun1, and shows subcellular co-localizations with Src1, Sun1, the ESCRT component CHMP7 and the IST1-like protein filactin, suggesting that the principal pathway of mitotic nuclear envelope remodeling is conserved between animals and Dictyostelium amoebae.
Keywords: ESCRT; LEM-domain; Spastin; dictyostelium; mitosis; nuclear envelope; sun1.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
Similar articles
-
Temporal Changes in Nuclear Envelope Permeability during Semi-Closed Mitosis in Dictyostelium Amoebae.Cells. 2023 May 13;12(10):1380. doi: 10.3390/cells12101380. Cells. 2023. PMID: 37408214 Free PMC article.
-
Nuclear envelope organization in Dictyostelium discoideum.Int J Dev Biol. 2019;63(8-9-10):509-519. doi: 10.1387/ijdb.190184rg. Int J Dev Biol. 2019. PMID: 31840788 Review.
-
Spastin and ESCRT-III coordinate mitotic spindle disassembly and nuclear envelope sealing.Nature. 2015 Jun 11;522(7555):231-5. doi: 10.1038/nature14408. Epub 2015 Jun 3. Nature. 2015. PMID: 26040712
-
Dictyostelium Sun1 is a dynamic membrane protein of both nuclear membranes and required for centrosomal association with clustered centromeres.Eur J Cell Biol. 2009 Nov;88(11):621-38. doi: 10.1016/j.ejcb.2009.06.003. Epub 2009 Jul 24. Eur J Cell Biol. 2009. PMID: 19632001
-
Remodeling the nuclear membrane during closed mitosis.Curr Opin Cell Biol. 2013 Feb;25(1):142-8. doi: 10.1016/j.ceb.2012.09.001. Epub 2012 Oct 5. Curr Opin Cell Biol. 2013. PMID: 23040820 Review.
Cited by
-
Temporal Changes in Nuclear Envelope Permeability during Semi-Closed Mitosis in Dictyostelium Amoebae.Cells. 2023 May 13;12(10):1380. doi: 10.3390/cells12101380. Cells. 2023. PMID: 37408214 Free PMC article.
References
-
- Batsios P, Gräf R, Koonce MP, et al. Nuclear envelope organization in Dictyostelium discoideum. Int J Dev Biol. 2019;63(8–9–10):509–519. - PubMed
-
- McIntosh JR, Roos UP, Neighbors B, et al. Architecture of the microtubule component of mitotic spindles from Dictyostelium discoideum. J Cell Sci. 1985;75(1):93–129. - PubMed
-
- Meyer I, Peter T, Batsios P, et al. CP39, CP75 and CP91 are major structural components of the Dictyostelium centrosome’s core structure. Eur J Cell Biol. 2017;96(2):119–130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
