Mediators of Neuropathic Pain; Focus on Spinal Microglia, CSF-1, BDNF, CCL21, TNF-α, Wnt Ligands, and Interleukin 1β
- PMID: 35295524
- PMCID: PMC8915739
- DOI: 10.3389/fpain.2021.698157
Mediators of Neuropathic Pain; Focus on Spinal Microglia, CSF-1, BDNF, CCL21, TNF-α, Wnt Ligands, and Interleukin 1β
Abstract
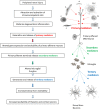
Intractable neuropathic pain is a frequent consequence of nerve injury or disease. When peripheral nerves are injured, damaged axons undergo Wallerian degeneration. Schwann cells, mast cells, fibroblasts, keratinocytes and epithelial cells are activated leading to the generation of an "inflammatory soup" containing cytokines, chemokines and growth factors. These primary mediators sensitize sensory nerve endings, attract macrophages, neutrophils and lymphocytes, alter gene expression, promote post-translational modification of proteins, and alter ion channel function in primary afferent neurons. This leads to increased excitability and spontaneous activity and the generation of secondary mediators including colony stimulating factor 1 (CSF-1), chemokine C-C motif ligand 21 (CCL-21), Wnt3a, and Wnt5a. Release of these mediators from primary afferent neurons alters the properties of spinal microglial cells causing them to release tertiary mediators, in many situations via ATP-dependent mechanisms. Tertiary mediators such as BDNF, tumor necrosis factor α (TNF-α), interleukin 1β (IL-1β), and other Wnt ligands facilitate the generation and transmission of nociceptive information by increasing excitatory glutamatergic transmission and attenuating inhibitory GABA and glycinergic transmission in the spinal dorsal horn. This review focusses on activation of microglia by secondary mediators, release of tertiary mediators from microglia and a description of their actions in the spinal dorsal horn. Attention is drawn to the substantial differences in the precise roles of various mediators in males compared to females. At least 25 different mediators have been identified but the similarity of their actions at sensory nerve endings, in the dorsal root ganglia and in the spinal cord means there is considerable redundancy in the available mechanisms. Despite this, behavioral studies show that interruption of the actions of any single mediator can relieve signs of pain in experimental animals. We draw attention this paradox. It is difficult to explain how inactivation of one mediator can relieve pain when so many parallel pathways are available.
Keywords: central sensitization; chemokine; cytokine; dorsal horn; growth factor; nerve injury; neuropathy; synaptic transmission.
Copyright © 2021 Boakye, Tang and Smith.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Receptor dependence of BDNF actions in superficial dorsal horn: relation to central sensitization and actions of macrophage colony stimulating factor 1.J Neurophysiol. 2019 Jun 1;121(6):2308-2322. doi: 10.1152/jn.00839.2018. Epub 2019 Apr 17. J Neurophysiol. 2019. PMID: 30995156
-
BDNF in Neuropathic Pain; the Culprit that Cannot be Apprehended.Neuroscience. 2024 Apr 5;543:49-64. doi: 10.1016/j.neuroscience.2024.02.020. Epub 2024 Feb 28. Neuroscience. 2024. PMID: 38417539 Review.
-
The Known Biology of Neuropathic Pain and Its Relevance to Pain Management.Can J Neurol Sci. 2024 Jan;51(1):32-39. doi: 10.1017/cjn.2023.10. Epub 2023 Feb 17. Can J Neurol Sci. 2024. PMID: 36799022 Review.
-
TNF-α Differentially Regulates Synaptic Plasticity in the Hippocampus and Spinal Cord by Microglia-Dependent Mechanisms after Peripheral Nerve Injury.J Neurosci. 2017 Jan 25;37(4):871-881. doi: 10.1523/JNEUROSCI.2235-16.2016. J Neurosci. 2017. PMID: 28123022 Free PMC article.
-
Minocycline, a microglial inhibitor, blocks spinal CCL2-induced heat hyperalgesia and augmentation of glutamatergic transmission in substantia gelatinosa neurons.J Neuroinflammation. 2014 Jan 10;11:7. doi: 10.1186/1742-2094-11-7. J Neuroinflammation. 2014. PMID: 24405660 Free PMC article.
Cited by
-
The role of non-coding RNAs in neuropathic pain.Pflugers Arch. 2024 Nov;476(11):1625-1643. doi: 10.1007/s00424-024-02989-y. Epub 2024 Jul 17. Pflugers Arch. 2024. PMID: 39017932 Review.
-
Interactions and Trends of Interleukins, PAI-1, CRP, and TNF-α in Inflammatory Responses during the Perioperative Period of Joint Arthroplasty: Implications for Pain Management-A Narrative Review.J Pers Med. 2024 May 17;14(5):537. doi: 10.3390/jpm14050537. J Pers Med. 2024. PMID: 38793119 Free PMC article. Review.
-
Impact of inflammation and Treg cell regulation on neuropathic pain in spinal cord injury: mechanisms and therapeutic prospects.Front Immunol. 2024 Jan 29;15:1334828. doi: 10.3389/fimmu.2024.1334828. eCollection 2024. Front Immunol. 2024. PMID: 38348031 Free PMC article. Review.
-
Sigma-1 Receptor Inhibition Reduces Mechanical Allodynia and Modulate Neuroinflammation in Chronic Neuropathic Pain.Mol Neurobiol. 2024 May;61(5):2672-2685. doi: 10.1007/s12035-023-03717-w. Epub 2023 Nov 3. Mol Neurobiol. 2024. PMID: 37922065 Free PMC article.
-
Research Progress on the Mechanisms of Central Post-Stroke Pain: A Review.Cell Mol Neurobiol. 2023 Oct;43(7):3083-3098. doi: 10.1007/s10571-023-01360-6. Epub 2023 May 11. Cell Mol Neurobiol. 2023. PMID: 37166685 Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous