Engineering stem cells to produce exosomes with enhanced bone regeneration effects: an alternative strategy for gene therapy
- PMID: 35292020
- PMCID: PMC8922796
- DOI: 10.1186/s12951-022-01347-3
Engineering stem cells to produce exosomes with enhanced bone regeneration effects: an alternative strategy for gene therapy
Abstract
Background: Exosomes derived from stem cells have been widely studied for promoting regeneration and reconstruction of multiple tissues as "cell-free" therapies. However, the applications of exosomes have been hindered by limited sources and insufficient therapeutic potency.
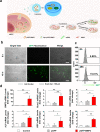
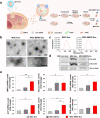
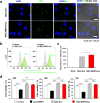
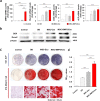
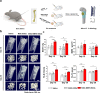
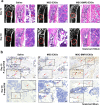
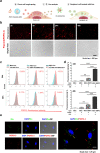
Results: In this study, a stem cell-mediated gene therapy strategy is developed in which mediator mesenchymal stem cells are genetically engineered by bone morphogenetic protein-2 gene to produce exosomes (MSC-BMP2-Exo) with enhanced bone regeneration potency. This effect is attributed to the synergistic effect of the content derived from MSCs and the up-regulated BMP2 gene expression. The MSC-BMP2-Exo also present homing ability to the injured site. The toxic effect of genetical transfection vehicles is borne by mediator MSCs, while the produced exosomes exhibit excellent biocompatibility. In addition, by plasmid tracking, it is interesting to find a portion of plasmid DNA can be encapsulated by exosomes and delivered to recipient cells.
Conclusions: In this strategy, engineered MSCs function as cellular factories, which effectively produce exosomes with designed and enhanced therapeutic effects. The accelerating effect in bone healing and the good biocompatibility suggest the potential clinical application of this strategy.
Keywords: Cell-free therapy; Exosomes; Gene therapy; Stem cell; Tissue regeneration.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Exosomes from conditioned media of bone marrow-derived mesenchymal stem cells promote bone regeneration by enhancing angiogenesis.PLoS One. 2019 Nov 21;14(11):e0225472. doi: 10.1371/journal.pone.0225472. eCollection 2019. PLoS One. 2019. PMID: 31751396 Free PMC article.
-
Functionally engineered extracellular vesicles improve bone regeneration.Acta Biomater. 2020 Jun;109:182-194. doi: 10.1016/j.actbio.2020.04.017. Epub 2020 Apr 16. Acta Biomater. 2020. PMID: 32305445 Free PMC article.
-
Mesenchymal stem cell exosomes enhance periodontal ligament cell functions and promote periodontal regeneration.Acta Biomater. 2019 Apr 15;89:252-264. doi: 10.1016/j.actbio.2019.03.021. Epub 2019 Mar 13. Acta Biomater. 2019. PMID: 30878447
-
The therapeutic potential of stem cell-derived exosomes in the ulcerative colitis and colorectal cancer.Stem Cell Res Ther. 2022 Apr 1;13(1):138. doi: 10.1186/s13287-022-02811-5. Stem Cell Res Ther. 2022. PMID: 35365226 Free PMC article. Review.
-
Stem Cell Mimicking Nanoencapsulation for Targeting Arthritis.Int J Nanomedicine. 2021 Dec 31;16:8485-8507. doi: 10.2147/IJN.S334298. eCollection 2021. Int J Nanomedicine. 2021. PMID: 35002240 Free PMC article. Review.
Cited by
-
Engineered mesenchymal stem cell-derived extracellular vesicles: A state-of-the-art multifunctional weapon against Alzheimer's disease.Theranostics. 2023 Feb 5;13(4):1264-1285. doi: 10.7150/thno.81860. eCollection 2023. Theranostics. 2023. PMID: 36923533 Free PMC article. Review.
-
Extracellular vesicles: a rising star for therapeutics and drug delivery.J Nanobiotechnology. 2023 Jul 20;21(1):231. doi: 10.1186/s12951-023-01973-5. J Nanobiotechnology. 2023. PMID: 37475025 Free PMC article. Review.
-
Application of stem cells in regeneration medicine.MedComm (2020). 2023 Jun 17;4(4):e291. doi: 10.1002/mco2.291. eCollection 2023 Aug. MedComm (2020). 2023. PMID: 37337579 Free PMC article. Review.
-
Stem cells and extracellular vesicles to improve preclinical orofacial soft tissue healing.Stem Cell Res Ther. 2023 Aug 15;14(1):203. doi: 10.1186/s13287-023-03423-3. Stem Cell Res Ther. 2023. PMID: 37580820 Free PMC article. Review.
-
Revolutionizing bone defect healing: the power of mesenchymal stem cells as seeds.Front Bioeng Biotechnol. 2024 Oct 21;12:1421674. doi: 10.3389/fbioe.2024.1421674. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39497791 Free PMC article. Review.
References
-
- Jiang S, Tian G, Yang Z, Gao X, Wang F, Li J, Tian Z, Huang B, Wei F, Sang X, Shao L, Zhou J, Wang Z, Liu S, Sui X, Guo Q, Guo W, Li X. Enhancement of acellular cartilage matrix scaffold by Wharton's jelly mesenchymal stem cell-derived exosomes to promote osteochondral regeneration. Bioact Mater. 2021;6:2711–2728. - PMC - PubMed
-
- Xu X, Liang Y, Li X, Ouyang K, Wang M, Cao T, Li W, Liu J, Xiong J, Li B, Xia J, Wang D, Duan L. Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials. 2021;269:120539. - PubMed
-
- Liu A, Lin D, Zhao H, Chen L, Cai B, Lin K, Shen SGF. Optimized BMSC-derived osteoinductive exosomes immobilized in hierarchical scaffold via lyophilization for bone repair through Bmpr2/Acvr2b competitive receptor-activated Smad pathway. Biomaterials. 2021;272:120718. - PubMed
MeSH terms
Grants and funding
- 81972071/National Natural Science Foundation of China
- 81802959/National Natural Science Foundation of China
- 2021A1515010191/Guangdong Basic and Applied Basic Research Foundation
- 2018A030313888/Guangdong Basic and Applied Basic Research Foundation
- JCYJ20210324102001003/Science and Technology Research Funding of Shenzhen
LinkOut - more resources
Full Text Sources

