Randomized Clinical Trial of High-Dose Rifampicin With or Without Levofloxacin Versus Standard of Care for Pediatric Tuberculous Meningitis: The TBM-KIDS Trial
- PMID: 35291004
- PMCID: PMC9617573
- DOI: 10.1093/cid/ciac208
Randomized Clinical Trial of High-Dose Rifampicin With or Without Levofloxacin Versus Standard of Care for Pediatric Tuberculous Meningitis: The TBM-KIDS Trial
Abstract
Background: Pediatric tuberculous meningitis (TBM) commonly causes death or disability. In adults, high-dose rifampicin may reduce mortality. The role of fluoroquinolones remains unclear. There have been no antimicrobial treatment trials for pediatric TBM.
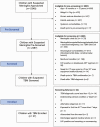
Methods: TBM-KIDS was a phase 2 open-label randomized trial among children with TBM in India and Malawi. Participants received isoniazid and pyrazinamide plus: (i) high-dose rifampicin (30 mg/kg) and ethambutol (R30HZE, arm 1); (ii) high-dose rifampicin and levofloxacin (R30HZL, arm 2); or (iii) standard-dose rifampicin and ethambutol (R15HZE, arm 3) for 8 weeks, followed by 10 months of standard treatment. Functional and neurocognitive outcomes were measured longitudinally using Modified Rankin Scale (MRS) and Mullen Scales of Early Learning (MSEL).
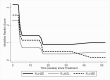
Results: Of 2487 children prescreened, 79 were screened and 37 enrolled. Median age was 72 months; 49%, 43%, and 8% had stage I, II, and III disease, respectively. Grade 3 or higher adverse events occurred in 58%, 55%, and 36% of children in arms 1, 2, and 3, with 1 death (arm 1) and 6 early treatment discontinuations (4 in arm 1, 1 each in arms 2 and 3). By week 8, all children recovered to MRS score of 0 or 1. Average MSEL scores were significantly better in arm 1 than arm 3 in fine motor, receptive language, and expressive language domains (P < .01).
Conclusions: In a pediatric TBM trial, functional outcomes were excellent overall. The trend toward higher frequency of adverse events but better neurocognitive outcomes in children receiving high-dose rifampicin requires confirmation in a larger trial.
Clinical trials registration: NCT02958709.
Keywords: clinical trial; high-dose rifampicin; levofloxacin; neuropsychological; pediatric tuberculous meningitis.
© The Author(s) 2022. Published by Oxford University Press for the Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. A. G. reports funding received by her institution outside the scope of this work from NIH, Unitaid, the Centers for Disease Control and Prevention (CDC), and various foundations (Wyncote, Ujala, Todi); participation on a data safety monitoring board or advisory board for the NIH/NIAID Advisory Council and Indo US Science Technology Governing Board; and a leadership or fiduciary role on the IMPAACT Network TB Scientific Committee and the World Health Organization (WHO) multidrug-resistant tuberculosis guidelines committee. D. B. D., K. Y. K., M. V., P. G., and S. B. report support outside the scope of this work given to the ICMR-NIRT for the TBM-KIDS trial by the NICHD and funded through John Hopkins University. K. T. T. reports grants or contracts (NIH, CDC), outside the scope of this work, and consulting fees from WHO. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
Similar articles
-
Safety and efficacy of levofloxacin versus rifampicin in tuberculous meningitis: an open-label randomized controlled trial.J Antimicrob Chemother. 2014 Aug;69(8):2246-51. doi: 10.1093/jac/dku103. Epub 2014 Apr 20. J Antimicrob Chemother. 2014. PMID: 24752957 Clinical Trial.
-
Intensified treatment with high dose rifampicin and levofloxacin compared to standard treatment for adult patients with tuberculous meningitis (TBM-IT): protocol for a randomized controlled trial.Trials. 2011 Feb 2;12:25. doi: 10.1186/1745-6215-12-25. Trials. 2011. PMID: 21288325 Free PMC article. Clinical Trial.
-
A Phase 2A Trial of the Safety and Tolerability of Increased Dose Rifampicin and Adjunctive Linezolid, With or Without Aspirin, for Human Immunodeficiency Virus-Associated Tuberculous Meningitis: The LASER-TBM Trial.Clin Infect Dis. 2023 Apr 17;76(8):1412-1422. doi: 10.1093/cid/ciac932. Clin Infect Dis. 2023. PMID: 36482216 Free PMC article. Clinical Trial.
-
High-dose rifampicin for the treatment of tuberculous meningitis: a meta-analysis of randomized controlled trials.J Clin Pharm Ther. 2022 Apr;47(4):445-454. doi: 10.1111/jcpt.13555. Epub 2021 Dec 12. J Clin Pharm Ther. 2022. PMID: 34897758 Review.
-
Tuberculous meningitis in children: Clinical management & outcome.Indian J Med Res. 2019 Aug;150(2):117-130. doi: 10.4103/ijmr.IJMR_786_17. Indian J Med Res. 2019. PMID: 31670267 Free PMC article. Review.
Cited by
-
Rifampicin and protein concentrations in paired spinal versus ventricular cerebrospinal fluid samples of children with tuberculous meningitis.J Antimicrob Chemother. 2024 Feb 1;79(2):280-286. doi: 10.1093/jac/dkad371. J Antimicrob Chemother. 2024. PMID: 38101948 Free PMC article.
-
Prenatal cannabinoid exposure and early language development.Front Pediatr. 2023 Nov 22;11:1290707. doi: 10.3389/fped.2023.1290707. eCollection 2023. Front Pediatr. 2023. PMID: 38078314 Free PMC article.
-
Advancing Diagnosis and Treatment in People Living with HIV and Tuberculosis Meningitis.Curr HIV/AIDS Rep. 2023 Dec;20(6):379-393. doi: 10.1007/s11904-023-00678-6. Epub 2023 Nov 10. Curr HIV/AIDS Rep. 2023. PMID: 37947980 Free PMC article. Review.
-
Twice-Daily Dosing of Dolutegravir in Infants on Rifampicin Treatment: A Pharmacokinetic Substudy of the EMPIRICAL Trial.Clin Infect Dis. 2024 Mar 20;78(3):702-710. doi: 10.1093/cid/ciad656. Clin Infect Dis. 2024. PMID: 37882611 Free PMC article. Clinical Trial.
-
Clinical standards for drug-susceptible TB in children and adolescents.Int J Tuberc Lung Dis. 2023 Aug 1;27(8):584-598. doi: 10.5588/ijtld.23.0085. Int J Tuberc Lung Dis. 2023. PMID: 37491754 Free PMC article.

