Spontaneous seizures in adult Fmr1 knockout mice: FVB.129P2-Pde6b+Tyrc-chFmr1tm1Cgr/J
- PMID: 35290907
- PMCID: PMC9050957
- DOI: 10.1016/j.eplepsyres.2022.106891
Spontaneous seizures in adult Fmr1 knockout mice: FVB.129P2-Pde6b+Tyrc-chFmr1tm1Cgr/J
Abstract
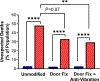
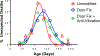
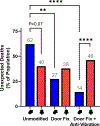
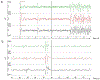
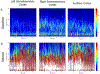
The prevalence of seizures in individuals with fragile X syndrome (FXS) is ~25%; however, there are no reports of spontaneous seizures in the Fmr1 knockout mouse model of FXS. Herein, we report that 48% of adult (median age P96), Fmr1 knockout mice from our colony were found expired in their home cages. We observed and recorded adult Fmr1 knockout mice having spontaneous convulsions in their home cages. In addition, we captured by electroencephalography an adult Fmr1 knockout mouse having a spontaneous seizure-during preictal, ictal, and postictal phases-which confirmed the presence of a generalized seizure. We did not observe this phenotype in control conspecifics or in juvenile (age <P35) Fmr1 knockout mice. We hypothesized that chronic, random, noise perturbations during development caused the phenotype. We recorded decibels (dB) in our vivarium. The average was 61 dB, but operating the automatic door to the vivarium caused spikes to 95 dB. We modified the door to eliminate noise spikes, which reduced unexpected deaths to 33% in Fmr1 knockout mice raised from birth in this environment (P = 0.07). As the modifications did not eliminate unexpected deaths, we further hypothesized that building vibrations may also be a contributing factor. After installing anti-vibration pads underneath housing carts, unexpected deaths of Fmr1 knockout mice born and raised in this environment decreased to 29% (P < 0.01 compared to the original environment). We also observed significant sex effects, for example, after interventions to reduce sound and vibration, significantly fewer male, but not female, Fmr1 knockout mice died unexpectedly (P < 0.001). The spontaneous seizure phenotype in our Fmr1 knockout mice could serve as a model of seizures observed in individuals with FXS, potentially offering a new translationally-valid phenotype for FXS research. Finally, these observations, although anomalous, serve as a reminder to consider gene-environment interactions when interpreting data derived from Fmr1 knockout mice.
Keywords: EEG; Fmr1; Fragile X syndrome; Generalized seizure; Sexual Dimorphism; Spontaneous seizure.
Copyright © 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





Similar articles
-
A phenotypic and molecular characterization of the fmr1-tm1Cgr fragile X mouse.Genes Brain Behav. 2004 Dec;3(6):337-59. doi: 10.1111/j.1601-183X.2004.00087.x. Genes Brain Behav. 2004. PMID: 15544577
-
ER stress-induced modulation of neural activity and seizure susceptibility is impaired in a fragile X syndrome mouse model.Neurobiol Dis. 2021 Oct;158:105450. doi: 10.1016/j.nbd.2021.105450. Epub 2021 Jul 23. Neurobiol Dis. 2021. PMID: 34303799 Free PMC article.
-
A single early-life seizure results in long-term behavioral changes in the adult Fmr1 knockout mouse.Epilepsy Res. 2019 Nov;157:106193. doi: 10.1016/j.eplepsyres.2019.106193. Epub 2019 Aug 29. Epilepsy Res. 2019. PMID: 31520894 Free PMC article.
-
Social behavior phenotypes in fragile X syndrome, autism, and the Fmr1 knockout mouse: theoretical comment on McNaughton et al. (2008).Behav Neurosci. 2008 Apr;122(2):483-9. doi: 10.1037/0735-7044.122.2.483. Behav Neurosci. 2008. PMID: 18410188 Review.
-
BDNF in fragile X syndrome.Neuropharmacology. 2014 Jan;76 Pt C:729-36. doi: 10.1016/j.neuropharm.2013.05.018. Epub 2013 May 29. Neuropharmacology. 2014. PMID: 23727436 Review.
Cited by
-
Epilepsy Characteristics in Neurodevelopmental Disorders: Research from Patient Cohorts and Animal Models Focusing on Autism Spectrum Disorder.Int J Mol Sci. 2022 Sep 16;23(18):10807. doi: 10.3390/ijms231810807. Int J Mol Sci. 2022. PMID: 36142719 Free PMC article. Review.
-
Improving Reproducibility to Enhance Scientific Rigor through Consideration of Mouse Diet.Animals (Basel). 2022 Dec 7;12(24):3448. doi: 10.3390/ani12243448. Animals (Basel). 2022. PMID: 36552368 Free PMC article.
-
FPT, a 2-Aminotetralin, Is a Potent Serotonin 5-HT1A, 5-HT1B, and 5-HT1D Receptor Agonist That Modulates Cortical Electroencephalogram Activity in Adult Fmr1 Knockout Mice.ACS Chem Neurosci. 2022 Dec 21;13(24):3629-3640. doi: 10.1021/acschemneuro.2c00574. Epub 2022 Dec 6. ACS Chem Neurosci. 2022. PMID: 36473166 Free PMC article.
-
Mouse models of fragile X-related disorders.Dis Model Mech. 2023 Feb 1;16(2):dmm049485. doi: 10.1242/dmm.049485. Epub 2023 Jan 24. Dis Model Mech. 2023. PMID: 36692473 Free PMC article. Review.
-
Activation of TLR7-mediated autophagy increases epileptic susceptibility via reduced KIF5A-dependent GABAA receptor transport in a murine model.Exp Mol Med. 2023 Jun;55(6):1159-1173. doi: 10.1038/s12276-023-01000-5. Epub 2023 Jun 1. Exp Mol Med. 2023. PMID: 37258573 Free PMC article.
References
-
- Ahn Y, Narous M, Tobias R, Rho JM, Mychasiuk R, 2014. The ketogenic diet modifies social and metabolic alterations identified in the prenatal valproic acid model of autism spectrum disorder. Dev Neurosci 36, 371–380. - PubMed
-
- Armstrong JL, Casey AB, Saraf TS, Mukherjee M, Booth RG, Canal CE, 2020. (S)-5-(2’-Fluorophenyl)-N,N-dimethyl-1,2,3,4-tetrahydronaphthalen-2-amine, a Serotonin Receptor Modulator, Possesses Anticonvulsant, Prosocial, and Anxiolytic-like Properties in an Fmr1 Knockout Mouse Model of Fragile X Syndrome and Autism Spectrum Disorder. ACS Pharmacol Transl Sci 3, 509–523. - PMC - PubMed
-
- Berry-Kravis E, 2002. Epilepsy in fragile X syndrome. Dev Med Child Neurol 44, 724–728. - PubMed
-
- Berry-Kravis E, Raspa M, Loggin-Hester L, Bishop E, Holiday D, Bailey DB, 2010. Seizures in Fragile X Syndrome: Characteristics and Comorbid Diagnoses. Ajidd-Am J Intellect 115, 461–472. - PubMed
-
- Boone CE, Davoudi H, Harrold JB, Foster DJ, 2018. Abnormal Sleep Architecture and Hippocampal Circuit Dysfunction in a Mouse Model of Fragile X Syndrome. Neuroscience 384, 275–289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

