Long-term efficacy and safety of subcutaneous concizumab prophylaxis in hemophilia A and hemophilia A/B with inhibitors
- PMID: 35290453
- PMCID: PMC9198939
- DOI: 10.1182/bloodadvances.2021006403
Long-term efficacy and safety of subcutaneous concizumab prophylaxis in hemophilia A and hemophilia A/B with inhibitors
Abstract
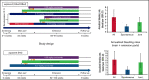
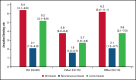
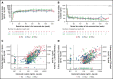
Despite current therapies, there remains an unmet need for treatment for patients with hemophilia. The main parts of two phase 2 trials established clinical proof-of-concept for once-daily, subcutaneous concizumab prophylaxis in patients with hemophilia A/B with inhibitors (HAwI/HBwI; explorer4) and severe hemophilia A without inhibitors (HA; explorer5). Here, we present results from extension parts of these trials, included to evaluate longer term safety and efficacy. Both trials included main (≥24 weeks) and extension (52-102 weeks) parts, with patients receiving concizumab 0.15 mg/kg with potential dose escalation to concizumab 0.20 or 0.25 mg/kg if they experienced ≥3 treated spontaneous bleeding episodes within 12 weeks. Endpoints included annualized bleeding rate (ABR), adverse events (AEs), and occurrence of antidrug antibodies. Thromboembolic events were AEs of special interest. Thirty-six patients with HA, 15 with HAwI, and 10 with HBwI were exposed to concizumab. Estimated ABRs during the main + extension parts at last dose level were 4.8 (95% confidence interval [CI], 3.2-7.2) and 6.4 (95% CI, 4.1-9.9) in explorer4 and explorer5, respectively (spontaneous ABRs were 1.8 [95% CI, 1.2-2.6] and 2.1 [95% CI, 1.3-3.3]). Most AEs were mild, with no deaths, events leading to withdrawal, or thromboembolic events. Anti-drug antibodies developed in 25% of patients and were low titer and transient, with no observed clinical effect in most cases. Results of the main + extension parts of these trials were consistent with results of the main parts. Ongoing phase 3 trials will further evaluate concizumab as a once-daily, subcutaneous treatment across hemophilia subtypes. These trials were registered at www.clinicaltrials.gov as #NCT03196284 and #NCT03196297.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures

Similar articles
-
Subcutaneous concizumab prophylaxis in hemophilia A and hemophilia A/B with inhibitors: phase 2 trial results.Blood. 2019 Nov 28;134(22):1973-1982. doi: 10.1182/blood.2019001542. Blood. 2019. PMID: 31444162 Free PMC article. Clinical Trial.
-
Phase 3 Trial of Concizumab in Hemophilia with Inhibitors.N Engl J Med. 2023 Aug 31;389(9):783-794. doi: 10.1056/NEJMoa2216455. N Engl J Med. 2023. PMID: 37646676 Clinical Trial.
-
Concizumab: a novel anti-TFPI therapeutic for hemophilia.Blood Adv. 2021 Jan 12;5(1):279. doi: 10.1182/bloodadvances.2019001140. Blood Adv. 2021. PMID: 33570646 Free PMC article.
-
Concizumab: First Approval.Drugs. 2023 Jul;83(11):1053-1059. doi: 10.1007/s40265-023-01912-6. Drugs. 2023. PMID: 37341887 Review.
-
New therapies using nonfactor products for patients with hemophilia and inhibitors.Blood. 2019 Jan 31;133(5):399-406. doi: 10.1182/blood-2018-07-820712. Epub 2018 Dec 17. Blood. 2019. PMID: 30559263 Review.
Cited by
-
Current status and future prospects of activated recombinant coagulation factor VIIa, NovoSeven®, in the treatment of haemophilia and rare bleeding disorders.Ann Hematol. 2024 Aug;103(8):2647-2658. doi: 10.1007/s00277-023-05287-2. Epub 2023 Jun 30. Ann Hematol. 2024. PMID: 37391649 Free PMC article. Review.
-
Therapeutic Fusion Proteins.AAPS J. 2023 Nov 30;26(1):3. doi: 10.1208/s12248-023-00873-8. AAPS J. 2023. PMID: 38036919 Review.
-
Hemophilia a patients with inhibitors: Mechanistic insights and novel therapeutic implications.Front Immunol. 2022 Dec 8;13:1019275. doi: 10.3389/fimmu.2022.1019275. eCollection 2022. Front Immunol. 2022. PMID: 36569839 Free PMC article. Review.
-
Non-clotting factor therapies for preventing bleeds in people with congenital hemophilia A or B.Cochrane Database Syst Rev. 2024 Feb 27;2(2):CD014544. doi: 10.1002/14651858.CD014544.pub2. Cochrane Database Syst Rev. 2024. PMID: 38411279 Review.
-
Hemophilia patients: are they naturally anticoagulated?Intern Emerg Med. 2023 Aug;18(5):1251-1254. doi: 10.1007/s11739-023-03331-7. Epub 2023 Jul 29. Intern Emerg Med. 2023. PMID: 37515677
References
-
- Srivastava A, Santagostino E, Dougall A, et al. . WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26(suppl 6): 1-158. - PubMed
-
- Berntorp E, Dolan G, Hay C, et al. . European retrospective study of real-life haemophilia treatment. Haemophilia. 2017;23(1):105-114. - PubMed
-
- van den Berg HM, Fischer K, Carcao M, et al. ; PedNet Study Group . Timing of inhibitor development in more than 1000 previously untreated patients with severe hemophilia A. Blood. 2019;134(3):317-320. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

