Mitochondrial glutathione peroxidase 4 is indispensable for photoreceptor development and survival in mice
- PMID: 35288190
- PMCID: PMC8980337
- DOI: 10.1016/j.jbc.2022.101824
Mitochondrial glutathione peroxidase 4 is indispensable for photoreceptor development and survival in mice
Abstract
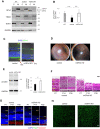
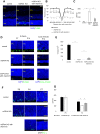
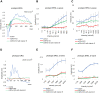
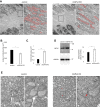
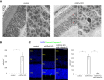
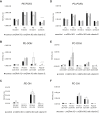
Glutathione peroxidase 4 (GPx4) is known for its unique function in the direct detoxification of lipid peroxides in the cell membrane and as a key regulator of ferroptosis, a form of lipid peroxidation-induced nonapoptotic cell death. However, the cytosolic isoform of GPx4 is considered to play a major role in inhibiting ferroptosis in somatic cells, whereas the roles of the mitochondrial isoform of GPx4 (mGPx4) in cell survival are not yet clear. In the present study, we found that mGPx4 KO mice exhibit a cone-rod dystrophy-like phenotype in which loss of cone photoreceptors precedes loss of rod photoreceptors. Specifically, in mGPx4 KO mice, cone photoreceptors disappeared prior to their maturation, whereas rod photoreceptors persisted through maturation but gradually degenerated afterward. Mechanistically, we demonstrated that vitamin E supplementation significantly ameliorated photoreceptor loss in these mice. Furthermore, LC-MS showed a significant increase in peroxidized phosphatidylethanolamine esterified with docosahexaenoic acid in the retina of mGPx4 KO mice. We also observed shrunken and uniformly condensed nuclei as well as caspase-3 activation in mGPx4 KO photoreceptors, suggesting that apoptosis was prevalent. Taken together, our findings indicate that mGPx4 is essential for the maturation of cone photoreceptors but not for the maturation of rod photoreceptors, although it is still critical for the survival of rod photoreceptors after maturation. In conclusion, we reveal novel functions of mGPx4 in supporting development and survival of photoreceptors in vivo.
Keywords: cell death; development; glutathione peroxidase 4; lipid peroxidation; mitochondria; phospholipid; photoreceptor; retina.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures
Similar articles
-
GPX4 in cell death, autophagy, and disease.Autophagy. 2023 Oct;19(10):2621-2638. doi: 10.1080/15548627.2023.2218764. Epub 2023 Jun 4. Autophagy. 2023. PMID: 37272058 Free PMC article. Review.
-
Glutathione peroxidase 4 is required for maturation of photoreceptor cells.J Biol Chem. 2012 Mar 2;287(10):7675-82. doi: 10.1074/jbc.M111.335174. Epub 2011 Dec 29. J Biol Chem. 2012. PMID: 22207760 Free PMC article.
-
Overexpression of SOD in retina: need for increase in H2O2-detoxifying enzyme in same cellular compartment.Free Radic Biol Med. 2011 Oct 1;51(7):1347-54. doi: 10.1016/j.freeradbiomed.2011.06.010. Epub 2011 Jul 5. Free Radic Biol Med. 2011. PMID: 21736939 Free PMC article.
-
The Leber congenital amaurosis protein, AIPL1, is needed for the viability and functioning of cone photoreceptor cells.Hum Mol Genet. 2010 Mar 15;19(6):1076-87. doi: 10.1093/hmg/ddp571. Epub 2009 Dec 30. Hum Mol Genet. 2010. PMID: 20042464 Free PMC article.
-
Lipid Peroxidation-Dependent Cell Death Regulated by GPx4 and Ferroptosis.Curr Top Microbiol Immunol. 2017;403:143-170. doi: 10.1007/82_2016_508. Curr Top Microbiol Immunol. 2017. PMID: 28204974 Review.
Cited by
-
Retinal pigment epithelium-specific ablation of GPx4 in adult mice recapitulates key features of geographic atrophy in age-related macular degeneration.Cell Death Dis. 2024 Oct 19;15(10):763. doi: 10.1038/s41419-024-07150-2. Cell Death Dis. 2024. PMID: 39426958 Free PMC article.
-
GPX4 in cell death, autophagy, and disease.Autophagy. 2023 Oct;19(10):2621-2638. doi: 10.1080/15548627.2023.2218764. Epub 2023 Jun 4. Autophagy. 2023. PMID: 37272058 Free PMC article. Review.
-
Crosstalk between regulated necrosis and micronutrition, bridged by reactive oxygen species.Front Nutr. 2022 Sep 23;9:1003340. doi: 10.3389/fnut.2022.1003340. eCollection 2022. Front Nutr. 2022. PMID: 36211509 Free PMC article. Review.
-
Non-Apoptotic Programmed Cell Death as Targets for Diabetic Retinal Neurodegeneration.Pharmaceuticals (Basel). 2024 Jun 26;17(7):837. doi: 10.3390/ph17070837. Pharmaceuticals (Basel). 2024. PMID: 39065688 Free PMC article. Review.
-
Research progress on the mechanism of ferroptosis and its role in diabetic retinopathy.Front Endocrinol (Lausanne). 2023 Jun 1;14:1155296. doi: 10.3389/fendo.2023.1155296. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37334304 Free PMC article. Review.
References
-
- Imai H., Hirao F., Sakamoto T., Sekine K., Mizukura Y., Saito M., Kitamoto T., Hayasaka M., Hanaoka K., Nakagawa Y. Early embryonic lethality caused by targeted disruption of the mouse PHGPx gene. Biochem. Biophys. Res. Commun. 2003;305:278–286. - PubMed
-
- Imai H., Matsuoka M., Kumagai T., Sakamoto T., Koumura T. In: Apoptotic and Non-apoptotic Cell Death. Nagata S., Nakano H., editors. Vol. 403. Current Topics in Microbiology and Immunology, Springer International Publishing; Cham: 2016. Lipid peroxidation-dependent cell death regulated by GPx4 and ferroptosis; pp. 143–170. - PubMed
-
- Imai H., Hakkaku N., Iwamoto R., Suzuki J., Suzuki T., Tajima Y., Konishi K., Minami S., Ichinose S., Ishizaka K., Shioda S., Arata S., Nishimura M., Naito S., Nakagawa Y. Depletion of selenoprotein GPx4 in spermatocytes causes male infertility in mice. J. Biol. Chem. 2009;284:32522–32532. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

