Engineering of Sulfolobus acidocaldarius for Hemicellulosic Biomass Utilization
- PMID: 35283427
- PMCID: PMC9628888
- DOI: 10.4014/jmb.2202.02016
Engineering of Sulfolobus acidocaldarius for Hemicellulosic Biomass Utilization
Abstract
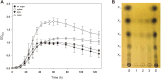
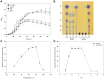
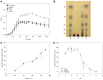
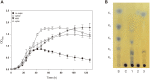
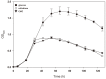
The saccharification of cellulose and hemicellulose is essential for utilizing lignocellulosic biomass as a biofuel. While cellulose is composed of glucose only, hemicelluloses are composed of diverse sugars such as xylose, arabinose, glucose, and galactose. Sulfolobus acidocaldarius is a good potential candidate for biofuel production using hemicellulose as this archaeon simultaneously utilizes various sugars. However, S. acidocaldarius has to be manipulated because the enzyme that breaks down hemicellulose is not present in this species. Here, we engineered S. acidocaldarius to utilize xylan as a carbon source by introducing xylanase and β-xylosidase. Heterologous expression of β-xylosidase enhanced the organism's degradability and utilization of xylooligosaccharides (XOS), but the mutant still failed to grow when xylan was provided as a carbon source. S. acidocaldarius exhibited the ability to degrade xylan into XOS when xylanase was introduced, but no further degradation proceeded after this sole reaction. Following cell growth and enzyme reaction, S. acidocaldarius successfully utilized xylan in the synergy between xylanase and β-xylosidase.
Keywords: Hyperthermophiles; Sulfolobus acidocaldarius; carbohydrateactive enzyme; hemicellulose.
Conflict of interest statement
The authors have no financial conflicts of interest to declare.
Figures
Similar articles
-
Absence of diauxie during simultaneous utilization of glucose and Xylose by Sulfolobus acidocaldarius.J Bacteriol. 2011 Mar;193(6):1293-301. doi: 10.1128/JB.01219-10. Epub 2011 Jan 14. J Bacteriol. 2011. PMID: 21239580 Free PMC article.
-
Modular Optimization of a Hemicellulose-Utilizing Pathway in Corynebacterium glutamicum for Consolidated Bioprocessing of Hemicellulosic Biomass.ACS Synth Biol. 2016 Apr 15;5(4):334-43. doi: 10.1021/acssynbio.5b00228. Epub 2016 Feb 8. ACS Synth Biol. 2016. PMID: 26808593
-
Sulfolobus acidocaldarius Transports Pentoses via a Carbohydrate Uptake Transporter 2 (CUT2)-Type ABC Transporter and Metabolizes Them through the Aldolase-Independent Weimberg Pathway.Appl Environ Microbiol. 2018 Jan 17;84(3):e01273-17. doi: 10.1128/AEM.01273-17. Print 2018 Feb 1. Appl Environ Microbiol. 2018. PMID: 29150511 Free PMC article.
-
Properties and applications of microbial beta-D-xylosidases featuring the catalytically efficient enzyme from Selenomonas ruminantium.Appl Microbiol Biotechnol. 2010 May;86(6):1647-58. doi: 10.1007/s00253-010-2538-y. Epub 2010 Mar 30. Appl Microbiol Biotechnol. 2010. PMID: 20352422 Review.
-
Insights into the mechanism of enzymatic hydrolysis of xylan.Appl Microbiol Biotechnol. 2016 Jun;100(12):5205-14. doi: 10.1007/s00253-016-7555-z. Epub 2016 Apr 25. Appl Microbiol Biotechnol. 2016. PMID: 27112349 Review.
References
-
- Martins F, Felgueiras C, Smitkova M, Caetano N. Analysis of fossil fuel energy consumption and environmental impacts in European countries. Energies. 2019;12:964. doi: 10.3390/en12060964. - DOI
-
- Nel WP, Cooper CJ. Implications of fossil fuel constraints on economic growth and global warming. Energy Policy. 2009;37:166–180. doi: 10.1016/j.enpol.2008.08.013. - DOI
-
- Gençsü I, Whitley S, Trilling M, van der Burg L, McLynn M, Worrall L. Phasing out public financial flows to fossil fuel production in Europe. Clim. Policy. 2020;20:1010–1023. doi: 10.1080/14693062.2020.1736978. - DOI

