Epigenetic Modifier Supplementation Improves Mitochondrial Respiration and Growth Rates and Alters DNA Methylation of Bovine Embryonic Fibroblast Cells Cultured in Divergent Energy Supply
- PMID: 35281844
- PMCID: PMC8907857
- DOI: 10.3389/fgene.2022.812764
Epigenetic Modifier Supplementation Improves Mitochondrial Respiration and Growth Rates and Alters DNA Methylation of Bovine Embryonic Fibroblast Cells Cultured in Divergent Energy Supply
Abstract
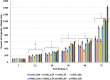
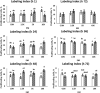
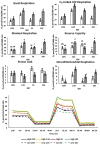
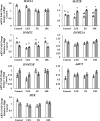
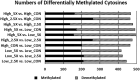
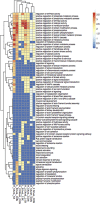
Epigenetic modifiers (EM; methionine, choline, folate, and vitamin B12) are important for early embryonic development due to their roles as methyl donors or cofactors in methylation reactions. Additionally, they are essential for the synthesis of nucleotides, polyamines, redox equivalents, and energy metabolites. Despite their importance, investigation into the supplementation of EM in ruminants has been limited to one or two epigenetic modifiers. Like all biochemical pathways, one-carbon metabolism needs to be stoichiometrically balanced. Thus, we investigated the effects of supplementing four EM encompassing the methionine-folate cycle on bovine embryonic fibroblast growth, mitochondrial function, and DNA methylation. We hypothesized that EM supplemented to embryonic fibroblasts cultured in divergent glucose media would increase mitochondrial respiration and cell growth rate and alter DNA methylation as reflected by changes in the gene expression of enzymes involved in methylation reactions, thereby improving the growth parameters beyond Control treated cells. Bovine embryonic fibroblast cells were cultured in Eagle's minimum essential medium with 1 g/L glucose (Low) or 4.5 g/L glucose (High). The control medium contained no additional OCM, whereas the treated media contained supplemented EM at 2.5, 5, and 10 times (×2.5, ×5, and ×10, respectively) the control media, except for methionine (limited to ×2). Therefore, the experimental design was a 2 (levels of glucose) × 4 (levels of EM) factorial arrangement of treatments. Cells were passaged three times in their respective treatment media before analysis for growth rate, cell proliferation, mitochondrial respiration, transcript abundance of methionine-folate cycle enzymes, and DNA methylation by reduced-representation bisulfite sequencing. Total cell growth was greatest in High ×10 and mitochondrial maximal respiration, and reserve capacity was greatest (p < 0.01) for High ×2.5 and ×10 compared with all other treatments. In Low cells, the total growth rate, mitochondrial maximal respiration, and reserve capacity increased quadratically to 2.5 and ×5 and decreased to control levels at ×10. The biological processes identified due to differential methylation included the positive regulation of GTPase activity, molecular function, protein modification processes, phosphorylation, and metabolic processes. These data are interpreted to imply that EM increased the growth rate and mitochondrial function beyond Control treated cells in both Low and High cells, which may be due to changes in the methylation of genes involved with growth and energy metabolism.
Keywords: DNA methylation; cell growth; embryonic fibroblasts; mitochondrial respiration; one-carbon metabolism.
Copyright © 2022 Crouse, Caton, Claycombe-Larson, Diniz, Lindholm-Perry, Reynolds, Dahlen, Borowicz and Ward.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
DNA methylation dataset of bovine embryonic fibroblast cells treated with epigenetic modifiers and divergent energy supply.Data Brief. 2022 Mar 22;42:108074. doi: 10.1016/j.dib.2022.108074. eCollection 2022 Jun. Data Brief. 2022. PMID: 35392625 Free PMC article.
-
Induced DNA hypomethylation by Folic Acid Deprivation in Bovine Fibroblast Donor Cells Improves Reprogramming of Somatic Cell Nuclear Transfer Embryos.Sci Rep. 2020 Mar 19;10(1):5076. doi: 10.1038/s41598-020-61797-3. Sci Rep. 2020. PMID: 32193457 Free PMC article.
-
Folate promotes S-adenosyl methionine reactions and the microbial methylation cycle and boosts ruminants production and reproduction.AMB Express. 2018 Apr 23;8(1):65. doi: 10.1186/s13568-018-0592-5. AMB Express. 2018. PMID: 29687201 Free PMC article. Review.
-
Impairments in SHMT2 expression or cellular folate availability reduce oxidative phosphorylation and pyruvate kinase activity.Genes Nutr. 2023 Mar 24;18(1):5. doi: 10.1186/s12263-023-00724-3. Genes Nutr. 2023. PMID: 36959541 Free PMC article.
-
Vitamin B12 , folate, and the methionine remethylation cycle-biochemistry, pathways, and regulation.J Inherit Metab Dis. 2019 Jul;42(4):673-685. doi: 10.1002/jimd.12009. Epub 2019 Jan 28. J Inherit Metab Dis. 2019. PMID: 30693532 Review.
Cited by
-
One-carbon metabolite supplementation increases vitamin B12, folate, and methionine cycle metabolites in beef heifers and fetuses in an energy dependent manner at day 63 of gestation.J Anim Sci. 2024 Jan 3;102:skae202. doi: 10.1093/jas/skae202. J Anim Sci. 2024. PMID: 39028746
-
One-carbon metabolite supplementation to heifers for the first 14 d of the estrous cycle alters the plasma and hepatic one-carbon metabolite pool and methionine-folate cycle enzyme transcript abundance in a dose-dependent manner.J Anim Sci. 2023 Jan 3;101:skac419. doi: 10.1093/jas/skac419. J Anim Sci. 2023. PMID: 36566452 Free PMC article.
-
Concentrations of vitamin B12 and folate in maternal serum and fetal fluids, metabolite interrelationships, and hepatic transcript abundance of key folate and methionine cycle genes: the impacts of maternal nutrition during the first 50 d of gestation.J Anim Sci. 2023 Jan 3;101:skad139. doi: 10.1093/jas/skad139. J Anim Sci. 2023. PMID: 37129588 Free PMC article.
-
Nitric oxide modulates folate-mediated one-carbon metabolism and mitochondrial energy levels of peaches during cold storage.Front Nutr. 2023 May 5;10:1184736. doi: 10.3389/fnut.2023.1184736. eCollection 2023. Front Nutr. 2023. PMID: 37215226 Free PMC article.
-
Effects of DNA Methylation on Gene Expression and Phenotypic Traits in Cattle: A Review.Int J Mol Sci. 2023 Jul 25;24(15):11882. doi: 10.3390/ijms241511882. Int J Mol Sci. 2023. PMID: 37569258 Free PMC article. Review.
References
-
- Alharthi A. S., Batistel F., Abdelmegeid M. K., Lascano G., Parys C., Helmbrecht A., et al. (2018). Maternal Supply of Methionine during Late-Pregnancy Enhances Rate of Holstein Calf Development In Utero and Postnatal Growth to a Greater Extent Than Colostrum Source. J. Anim. Sci Biotechnol 9 (1), 83. 10.1186/s40104-018-0298-1 - DOI - PMC - PubMed
-
- Alharthi A. S., Coleman D. N., Liang Y., Batistel F., Elolimy A. A., Yambao R. C., et al. (2019). Hepatic 1-carbon Metabolism Enzyme Activity, Intermediate Metabolites, and Growth in Neonatal Holstein Dairy Calves Are Altered by Maternal Supply of Methionine during Late Pregnancy. J. Dairy Sci. 102 (11), 10291–10303. 10.3168/jds.2019-16562 - DOI - PubMed
-
- Andrews S. (2010). FASTQC. A Quality Control Tool for for High Throughput Sequence Data. Available at: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ .
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

