Metabolism Characteristics of Th17 and Regulatory T Cells in Autoimmune Diseases
- PMID: 35281063
- PMCID: PMC8913504
- DOI: 10.3389/fimmu.2022.828191
Metabolism Characteristics of Th17 and Regulatory T Cells in Autoimmune Diseases
Abstract
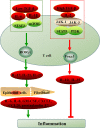
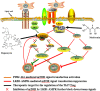
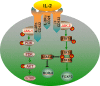
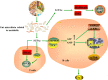
The abnormal number and functional deficiency of immune cells are the pathological basis of various diseases. Recent years, the imbalance of Th17/regulatory T (Treg) cell underlies the occurrence and development of inflammation in autoimmune diseases (AID). Currently, studies have shown that material and energy metabolism is essential for maintaining cell survival and normal functions and the altered metabolic state of immune cells exists in a variety of AID. This review summarizes the biology and functions of Th17 and Treg cells in AID, with emphasis on the advances of the roles and regulatory mechanisms of energy metabolism in activation, differentiation and physiological function of Th17 and Treg cells, which will facilitate to provide targets for the treatment of immune-mediated diseases.
Keywords: Th17 cells; autoimmune disease; gut microbiota; immunometabolism; regulatory T cells; single-cell metabolism.
Copyright © 2022 Qin, Gao and Luo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Inhibition of microRNA-155 ameliorates experimental autoimmune myocarditis by modulating Th17/Treg immune response.J Mol Med (Berl). 2016 Sep;94(9):1063-79. doi: 10.1007/s00109-016-1414-3. Epub 2016 Apr 6. J Mol Med (Berl). 2016. PMID: 27052830
-
MicroRNA-mediated regulation of T helper type 17/regulatory T-cell balance in autoimmune disease.Immunology. 2018 Dec;155(4):427-434. doi: 10.1111/imm.12994. Epub 2018 Sep 10. Immunology. 2018. PMID: 30133700 Free PMC article. Review.
-
Glutamine metabolism in Th17/Treg cell fate: applications in Th17 cell-associated diseases.Sci China Life Sci. 2021 Feb;64(2):221-233. doi: 10.1007/s11427-020-1703-2. Epub 2020 Jul 13. Sci China Life Sci. 2021. PMID: 32671630 Review.
-
Th17 and regulatory T cell balance in autoimmune and inflammatory diseases.Autoimmun Rev. 2014 Jun;13(6):668-77. doi: 10.1016/j.autrev.2013.12.004. Epub 2014 Jan 11. Autoimmun Rev. 2014. PMID: 24418308 Review.
-
Artesunate targets cellular metabolism to regulate the Th17/Treg cell balance.Inflamm Res. 2023 May;72(5):1037-1050. doi: 10.1007/s00011-023-01729-9. Epub 2023 Apr 6. Inflamm Res. 2023. PMID: 37024544 Review.
Cited by
-
The effects of low-dose IL-2 on Th17/Treg cell imbalance in primary biliary cholangitis mouse models.BMC Gastroenterol. 2024 Feb 26;24(1):87. doi: 10.1186/s12876-024-03176-0. BMC Gastroenterol. 2024. PMID: 38408917 Free PMC article.
-
Mitochondrial dysfunctions in T cells: focus on inflammatory bowel disease.Front Immunol. 2023 Sep 22;14:1219422. doi: 10.3389/fimmu.2023.1219422. eCollection 2023. Front Immunol. 2023. PMID: 37809060 Free PMC article. Review.
-
Yiqi Jiedu Xiaoying Decoction Improves Experimental Autoimmune Thyroiditis in Rats by Regulating Th17/Treg Cell Balance.Endocr Metab Immune Disord Drug Targets. 2024;24(10):1186-1196. doi: 10.2174/0118715303256311231122094516. Endocr Metab Immune Disord Drug Targets. 2024. PMID: 38317460
-
Identification of biomarkers by machine learning classifiers to assist diagnose rheumatoid arthritis-associated interstitial lung disease.Arthritis Res Ther. 2022 May 19;24(1):115. doi: 10.1186/s13075-022-02800-2. Arthritis Res Ther. 2022. PMID: 35590341 Free PMC article.
-
Muscle-derived microRNAs correlated with thigh lean mass gains during progressive resistance training in older adults.J Appl Physiol (1985). 2024 Aug 1;137(2):262-273. doi: 10.1152/japplphysiol.00680.2023. Epub 2024 Jun 27. J Appl Physiol (1985). 2024. PMID: 38932684
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

