A Novel Copper(II) Indenoisoquinoline Complex Inhibits Topoisomerase I, Induces G2 Phase Arrest, and Autophagy in Three Adenocarcinomas
- PMID: 35280788
- PMCID: PMC8908320
- DOI: 10.3389/fonc.2022.837373
A Novel Copper(II) Indenoisoquinoline Complex Inhibits Topoisomerase I, Induces G2 Phase Arrest, and Autophagy in Three Adenocarcinomas
Abstract
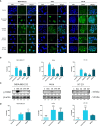
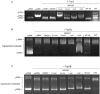
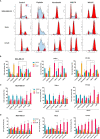
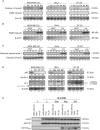
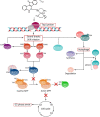
Topoisomerases, targets of inhibitors used in chemotherapy, induce DNA breaks accumulation leading to cancer cell death. A newly synthesized copper(II) indenoisoquinoline complex WN197 exhibits a cytotoxic effect below 0.5 µM, on MDA-MB-231, HeLa, and HT-29 cells. At low doses, WN197 inhibits topoisomerase I. At higher doses, it inhibits topoisomerase IIα and IIβ, and displays DNA intercalation properties. DNA damage is detected by the presence of γH2AX. The activation of the DNA Damage Response (DDR) occurs through the phosphorylation of ATM/ATR, Chk1/2 kinases, and the increase of p21, a p53 target. WN197 induces a G2 phase arrest characterized by the unphosphorylated form of histone H3, the accumulation of phosphorylated Cdk1, and an association of Cdc25C with 14.3.3. Cancer cells die by autophagy with Beclin-1 accumulation, LC3-II formation, p62 degradation, and RAPTOR phosphorylation in the mTOR complex. Finally, WN197 by inhibiting topoisomerase I at low concentration with high efficiency is a promising agent for the development of future DNA damaging chemotherapies.
Keywords: adenocarcinoma; autophagy; cell cycle; copper(II) complex; indenoisoquinoline; topoisomerase.
Copyright © 2022 Molinaro, Wambang, Bousquet, Vercoutter-Edouart, Pélinski, Cailliau and Martoriati.
Conflict of interest statement
Author NW was employed by AGAT Laboratories, Intertek. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
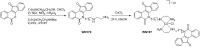


Similar articles
-
Synthesis and Biological Activity of a New Indenoisoquinoline Copper Derivative as a Topoisomerase I Inhibitor.Int J Mol Sci. 2023 Sep 26;24(19):14590. doi: 10.3390/ijms241914590. Int J Mol Sci. 2023. PMID: 37834037 Free PMC article.
-
Human topoisomerase II function, tyrosine phosphorylation and cell cycle checkpoints.Proc Soc Exp Biol Med. 1998 Mar;217(3):327-34. doi: 10.3181/00379727-217-44240. Proc Soc Exp Biol Med. 1998. PMID: 9492343 Review.
-
The Topoisomerase 1 Inhibitor Austrobailignan-1 Isolated from Koelreuteria henryi Induces a G2/M-Phase Arrest and Cell Death Independently of p53 in Non-Small Cell Lung Cancer Cells.PLoS One. 2015 Jul 6;10(7):e0132052. doi: 10.1371/journal.pone.0132052. eCollection 2015. PLoS One. 2015. PMID: 26147394 Free PMC article.
-
Inhibition of topoisomerase IIalpha and G2 cell cycle arrest by NK314, a novel benzo[c]phenanthridine currently in clinical trials.Mol Cancer Ther. 2007 May;6(5):1501-8. doi: 10.1158/1535-7163.MCT-06-0780. Mol Cancer Ther. 2007. PMID: 17513599
-
Regulation of the G2/M transition by p53.Oncogene. 2001 Apr 5;20(15):1803-15. doi: 10.1038/sj.onc.1204252. Oncogene. 2001. PMID: 11313928 Review.
Cited by
-
New Cu+2 Complexes with N-Sulfonamide Ligands: Potential Antitumor, Antibacterial, and Antioxidant Agents.Molecules. 2022 May 23;27(10):3338. doi: 10.3390/molecules27103338. Molecules. 2022. PMID: 35630815 Free PMC article.
-
Potential of Copper and Copper Compounds for Anticancer Applications.Pharmaceuticals (Basel). 2023 Feb 3;16(2):234. doi: 10.3390/ph16020234. Pharmaceuticals (Basel). 2023. PMID: 37259382 Free PMC article. Review.
-
Synthesis and Biological Activity of a New Indenoisoquinoline Copper Derivative as a Topoisomerase I Inhibitor.Int J Mol Sci. 2023 Sep 26;24(19):14590. doi: 10.3390/ijms241914590. Int J Mol Sci. 2023. PMID: 37834037 Free PMC article.
-
Epigenetic modulations in cancer: predictive biomarkers and potential targets for overcoming the resistance to topoisomerase I inhibitors.Ann Med. 2023 Dec;55(1):2203946. doi: 10.1080/07853890.2023.2203946. Ann Med. 2023. PMID: 37092854 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

