Ionizing Radiation-Induced GDF15 Promotes Angiogenesis in Human Glioblastoma Models by Promoting VEGFA Expression Through p-MAPK1/SP1 Signaling
- PMID: 35280749
- PMCID: PMC8913883
- DOI: 10.3389/fonc.2022.801230
Ionizing Radiation-Induced GDF15 Promotes Angiogenesis in Human Glioblastoma Models by Promoting VEGFA Expression Through p-MAPK1/SP1 Signaling
Abstract
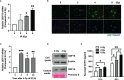
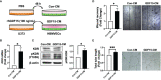
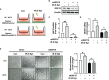
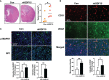
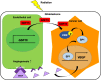
Glioblastoma multiforme (GBM), the most aggressive cancer type that has a poor prognosis, is characterized by enhanced and aberrant angiogenesis. In addition to surgical resection and chemotherapy, radiotherapy is commonly used to treat GBM. However, radiation-induced angiogenesis in GBM remains unexplored. This study examined the role of radiation-induced growth/differentiation factor-15 (GDF15) in regulating tumor angiogenesis by promoting intercellular cross-talk between brain endothelial cells (ECs) and glioblastoma cells. Radiation promoted GDF15 secretion from human brain microvascular endothelial cells (HBMVECs). Subsequently, GDF15 activated the transcriptional promoter VEGFA in the human glioblastoma cell line U373 through p-MAPK1/SP1 signaling. Upregulation of vascular endothelial growth factor (VEGF) expression in U373 cells resulted in the activation of angiogenic activity in HBMVECs via KDR phosphorylation. Wound healing, tube formation, and invasion assay results revealed that the conditioned medium of recombinant human GDF15 (rhGDF15)-stimulated U373 cell cultures promoted the angiogenic activity of HBMVECs. In the HBMVEC-U373 cell co-culture, GDF15 knockdown mitigated radiation-induced VEGFA upregulation in U373 cells and enhanced angiogenic activity of HBMVECs. Moreover, injecting rhGDF15-stimulated U373 cells into orthotopic brain tumors in mice promoted angiogenesis in the tumors. Thus, radiation-induced GDF15 is essential for the cross-talk between ECs and GBM cells and promotes angiogenesis. These findings indicate that GDF15 is a putative therapeutic target for patients with GBM undergoing radio-chemotherapy.
Keywords: GDF15; angiogenesis; endothelial cells; glioblastoma; radiotherapy.
Copyright © 2022 Park, Nam, Lee and Kim.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Distinct response to GDF15 knockdown in pediatric and adult glioblastoma cell lines.J Neurooncol. 2018 Aug;139(1):51-60. doi: 10.1007/s11060-018-2853-1. Epub 2018 Apr 18. J Neurooncol. 2018. PMID: 29671197
-
Loss of endothelial programmed cell death 10 activates glioblastoma cells and promotes tumor growth.Neuro Oncol. 2016 Apr;18(4):538-48. doi: 10.1093/neuonc/nov155. Epub 2015 Aug 8. Neuro Oncol. 2016. PMID: 26254477 Free PMC article.
-
PSMB8 inhibition decreases tumor angiogenesis in glioblastoma through vascular endothelial growth factor A reduction.Cancer Sci. 2020 Nov;111(11):4142-4153. doi: 10.1111/cas.14625. Epub 2020 Sep 8. Cancer Sci. 2020. PMID: 32816328 Free PMC article.
-
Multiprong control of glioblastoma multiforme invasiveness: blockade of pro-inflammatory signaling, anti-angiogenesis, and homeostasis restoration.Cancer Metastasis Rev. 2021 Sep;40(3):643-647. doi: 10.1007/s10555-021-09987-x. Cancer Metastasis Rev. 2021. PMID: 34519960 Free PMC article. Review.
-
Overview of Transforming Growth Factor β Superfamily Involvement in Glioblastoma Initiation and Progression.Asian Pac J Cancer Prev. 2015;16(16):6813-23. doi: 10.7314/apjcp.2015.16.16.6813. Asian Pac J Cancer Prev. 2015. PMID: 26514451 Review.
Cited by
-
Overexpression of Growth Differentiation Factor 15 in Glioblastoma Stem Cells Promotes Their Radioresistance.Cancers (Basel). 2023 Dec 20;16(1):27. doi: 10.3390/cancers16010027. Cancers (Basel). 2023. PMID: 38201456 Free PMC article.
-
Increased levels of a subset of angiogenesis-related plasma proteins in essential thrombocythemia.Ups J Med Sci. 2023 Mar 27;128. doi: 10.48101/ujms.v128.9194. eCollection 2023. Ups J Med Sci. 2023. PMID: 37051288 Free PMC article.
-
Growth differentiation factor 15 (GDF15) predicts relapse free and overall survival in unresected locally advanced non-small cell lung cancer treated with chemoradiotherapy.Radiat Oncol. 2024 Nov 7;19(1):155. doi: 10.1186/s13014-024-02546-y. Radiat Oncol. 2024. PMID: 39511611 Free PMC article. Clinical Trial.
-
Brucea javanica Seed Oil Emulsion and Shengmai Injections Improve Peripheral Microcirculation in Treatment of Gastric Cancer.Chin J Integr Med. 2024 Sep 3. doi: 10.1007/s11655-024-4103-z. Online ahead of print. Chin J Integr Med. 2024. PMID: 39225883
-
Antitumoral Activity of Molecular Hydrogen and Proton in the Treatment of Glioblastoma: An Atypical Pharmacology?Brain Sci. 2023 Aug 5;13(8):1168. doi: 10.3390/brainsci13081168. Brain Sci. 2023. PMID: 37626524 Free PMC article.
References
-
- Bulnes S, Bengoetxea H, Ortuzar N, Argandona EG, Garcia-Blanco A, Rico-Barrio I, et al. . Angiogenic Signalling Pathways Altered in Gliomas: Selection Mechanisms for More Aggressive Neoplastic Subpopulations With Invasive Phenotype. J Signal Transduct (2012) 2012:597915. doi: 10.1155/2012/597915 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

