Beyond depression and anxiety; a systematic review about the role of corticotropin-releasing hormone antagonists in diseases of the pelvic and abdominal organs
- PMID: 35275963
- PMCID: PMC8916623
- DOI: 10.1371/journal.pone.0264909
Beyond depression and anxiety; a systematic review about the role of corticotropin-releasing hormone antagonists in diseases of the pelvic and abdominal organs
Abstract
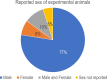
Evidence for beneficial effects of corticotropin releasing hormone (CRH) antagonists in abdominal and pelvic organs is emerging in preclinical studies. Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement a compilation of preclinical studies using CRH receptor antagonists as a treatment for abdominal and pelvic disease was carried out. The Animal Research: Reporting of In Vivo Experiments (ARRIVE) essential 10 guidelines were used to determine quality of the included studies. A total of 40 studies from the last 15 years studying irritable bowel syndrome, inflammatory bowel disease, endometriosis, enteritis, stress impact on gastrointestinal processes and exogenous CRH administration effects were included. Blockage of the CRH receptor 1 was mainly associated with beneficial effects while that of CRH receptor 2 worsened studied effects. However, time of administration, route of administration and the animal model used, all had an impact on the beneficial outcomes. Frequency of drugs administered indicated that astressin-2B, astressin and antalarmin were among the most utilized antagonists. Of concern, studies included were predominantly carried out in male models only, representing a gender discrepancy in preclinical studies compared to the clinical scenario. The ARRIVE score average was 13 with ~60% of the studies failing to randomize or blind the experimental units. Despite the failure to date of the CRH antagonists in moving across the clinical trials pipeline, there is evidence for their beneficial effects beyond mood disorders. Future pre-clinical studies should be tailored towards effectively predicting the clinical scenario, including reduction of bias and randomization.
Conflict of interest statement
We have read the journal’s policy and the authors of this manuscript have the following competing interests: ATR and CBA are co-founders of Sur180 Therapeutics, LLC. However, Sur 180 Therapeutics did not provide funding for the current study. ATR and CBA are named as co-inventors in the patent US-10,729,693 entitled, “Compositions and methods for the treatment of endometriosis”. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

Similar articles
-
Corticotropin-releasing hormone antagonists, astressin B and antalarmin: differing profiles of activity in rhesus monkeys.Neuropsychopharmacology. 2004 Jun;29(6):1112-21. doi: 10.1038/sj.npp.1300410. Neuropsychopharmacology. 2004. PMID: 14997174
-
Nonpeptide corticotropin-releasing hormone receptor type 1 antagonists and their applications in psychosomatic disorders.Neuroendocrinology. 2004;80(2):111-23. doi: 10.1159/000081785. Epub 2004 Oct 27. Neuroendocrinology. 2004. PMID: 15523186 Review.
-
Central CRH system in depression and anxiety--evidence from clinical studies with CRH1 receptor antagonists.Eur J Pharmacol. 2008 Apr 7;583(2-3):350-7. doi: 10.1016/j.ejphar.2007.12.032. Epub 2008 Jan 24. Eur J Pharmacol. 2008. PMID: 18272149 Review.
-
Effects of corticotropin-releasing hormone (CRH) on monocyte function, mediated by CRH-receptor subtype R1 and R2: a potential link between mood disorders and endothelial dysfunction?J Cardiovasc Pharmacol. 2006 Jan;47(1):110-6. doi: 10.1097/01.fjc.0000196240.58641.d3. J Cardiovasc Pharmacol. 2006. PMID: 16424794
-
The role of central corticotropin-releasing hormone in the anorexic and endocrine effects of the bacterial T cell superantigen, Staphylococcal enterotoxin A.Brain Behav Immun. 2005 Mar;19(2):138-46. doi: 10.1016/j.bbi.2004.06.002. Brain Behav Immun. 2005. PMID: 15664786
Cited by
-
Research Progress of Central and Peripheral Corticotropin-Releasing Hormone in Irritable Bowel Syndrome with Comorbid Dysthymic Disorders.Gut Liver. 2024 May 15;18(3):391-403. doi: 10.5009/gnl220346. Epub 2023 Aug 8. Gut Liver. 2024. PMID: 37551453 Free PMC article. Review.
-
Metabolic Adverse Effects of Psychotropic Drug Therapy: A Systematic Review.Eur J Investig Health Psychol Educ. 2023 Aug 12;13(8):1505-1520. doi: 10.3390/ejihpe13080110. Eur J Investig Health Psychol Educ. 2023. PMID: 37623307 Free PMC article. Review.
-
Design, Synthesis, and Biological Evaluations of Novel Thiazolo[4,5-d]pyrimidine Corticotropin Releasing Factor (CRF) Receptor Antagonists as Potential Treatments for Stress Related Disorders and Congenital Adrenal Hyperplasia (CAH).Molecules. 2024 Aug 1;29(15):3647. doi: 10.3390/molecules29153647. Molecules. 2024. PMID: 39125051 Free PMC article.
-
Corticotropin-Releasing Hormone: Biology and Therapeutic Opportunities.Biology (Basel). 2022 Dec 8;11(12):1785. doi: 10.3390/biology11121785. Biology (Basel). 2022. PMID: 36552294 Free PMC article.
-
The Role of Neuro-Immune Interaction in Chronic Pain Conditions; Functional Somatic Syndrome, Neurogenic Inflammation, and Peripheral Neuropathy.Int J Mol Sci. 2022 Aug 2;23(15):8574. doi: 10.3390/ijms23158574. Int J Mol Sci. 2022. PMID: 35955708 Free PMC article. Review.
References
-
- Nezi M, Mastorakos G, Mouslech Z. Corticotropin releasing hormone and the immune/inflammatory response. In: De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, et al.., editors. South Dartmouth (MA); 2000. - PubMed
-
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science (80-). 1981/09/18. 1981;213: 1394–1397. Available: http://www.ncbi.nlm.nih.gov/pubmed/6267699. doi: 10.1126/science.6267699 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

