Competition for refueling rather than cyclic reentry initiation evident in germinal centers
- PMID: 35275753
- PMCID: PMC7614495
- DOI: 10.1126/sciimmunol.abm0775
Competition for refueling rather than cyclic reentry initiation evident in germinal centers
Abstract
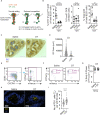
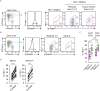
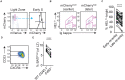
Antibody affinity maturation occurs in germinal centers (GCs) through iterative rounds of somatic hypermutation and proliferation in dark zones (DZs) and selection in light zones (LZs). GC B cells exit cell cycle a number of hours before entering LZs; therefore, continued participation in responses requires that they subsequently reenter cell cycle and move back to DZs, a process known as cyclic reentry. Affinity enhancements are thought to arise by B cells having to compete to initiate cyclic reentry each time they enter LZs, with T cell help being a major determinant; however, direct proof is lacking. Using Fucci2 mice, we confirmed an association between B cell receptor affinity and the first step of cyclic reentry, S phase initiation from a resting LZ state. However, neither T cell ablation nor MHCII deletion prevented resting LZ cells from reentering cell cycle, and this late G1-S transition was also not detectably restricted by competition. In contrast, using BATF induction as exemplar, we found that T cells "refueled" LZ cells in an affinity-dependent manner that was limited by both competition and cells' intrinsic antigen-acquiring abilities. Therefore, cyclic reentry initiation and B cell refueling are independently regulated in GCs, which may contribute to permitting cells of different competencies to be sustained alongside each other and allow T cell support to be provided across a dynamic range commensurate with affinity. We speculate that this less binary selection mechanism could help GCs nurture complex antibody maturation pathways and support the clonal diversity required for countering fast-evolving pathogens.
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Germinal Center B Cells Replace Their Antigen Receptors in Dark Zones and Fail Light Zone Entry when Immunoglobulin Gene Mutations are Damaging.Immunity. 2018 Sep 18;49(3):477-489.e7. doi: 10.1016/j.immuni.2018.08.025. Immunity. 2018. PMID: 30231983 Free PMC article.
-
Permissive selection followed by affinity-based proliferation of GC light zone B cells dictates cell fate and ensures clonal breadth.Proc Natl Acad Sci U S A. 2021 Jan 12;118(2):e2016425118. doi: 10.1073/pnas.2016425118. Proc Natl Acad Sci U S A. 2021. PMID: 33419925 Free PMC article.
-
Germinal Center Selection and Affinity Maturation Require Dynamic Regulation of mTORC1 Kinase.Immunity. 2017 Jun 20;46(6):1045-1058.e6. doi: 10.1016/j.immuni.2017.06.005. Immunity. 2017. PMID: 28636954 Free PMC article.
-
Germinal center responses to complex antigens.Immunol Rev. 2018 Jul;284(1):42-50. doi: 10.1111/imr.12661. Immunol Rev. 2018. PMID: 29944756 Free PMC article. Review.
-
Heterogeneity of germinal center B cells: New insights from single-cell studies.Eur J Immunol. 2021 Nov;51(11):2555-2567. doi: 10.1002/eji.202149235. Epub 2021 Aug 8. Eur J Immunol. 2021. PMID: 34324199 Review.
Cited by
-
'Persistent germinal center responses: slow-growing trees bear the best fruits'.Curr Opin Immunol. 2023 Aug;83:102332. doi: 10.1016/j.coi.2023.102332. Epub 2023 May 5. Curr Opin Immunol. 2023. PMID: 37150126 Free PMC article. Review.
-
High affinity mAb infusion can enhance maximum affinity maturation during HIV Env immunization.iScience. 2024 Mar 11;27(4):109495. doi: 10.1016/j.isci.2024.109495. eCollection 2024 Apr 19. iScience. 2024. PMID: 38550978 Free PMC article.
-
Regulation of BCR-mediated Ca2+ mobilization by MIZ1-TMBIM4 safeguards IgG1+ GC B cell-positive selection.Sci Immunol. 2024 Apr 5;9(94):eadk0092. doi: 10.1126/sciimmunol.adk0092. Epub 2024 Apr 5. Sci Immunol. 2024. PMID: 38579014 Free PMC article.
-
T-cell help in the germinal center: homing in on the role of IL-21.Int Immunol. 2024 Feb 21;36(3):89-98. doi: 10.1093/intimm/dxad056. Int Immunol. 2024. PMID: 38164992 Free PMC article.
-
IL-21 shapes germinal center polarization via light zone B cell selection and cyclin D3 upregulation.J Exp Med. 2023 Oct 2;220(10):e20221653. doi: 10.1084/jem.20221653. Epub 2023 Jul 19. J Exp Med. 2023. PMID: 37466652 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

