The Prescription Characteristics, Efficacy and Safety of Spironolactone in Real-World Patients With Acute Heart Failure Syndrome: A Prospective Nationwide Cohort Study
- PMID: 35274010
- PMCID: PMC8902170
- DOI: 10.3389/fcvm.2022.791446
The Prescription Characteristics, Efficacy and Safety of Spironolactone in Real-World Patients With Acute Heart Failure Syndrome: A Prospective Nationwide Cohort Study
Erratum in
-
Corrigendum: The Prescription Characteristics, Efficacy and Safety of Spironolactone in Real-World Patients With Acute Heart Failure Syndrome: A Prospective Nationwide Cohort Study.Front Cardiovasc Med. 2022 Apr 4;9:888829. doi: 10.3389/fcvm.2022.888829. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35479279 Free PMC article.
Abstract
Background: Randomized clinical trials of spironolactone showed significant mortality reduction in patients with heart failure with reduced ejection fraction. However, its role in acute heart failure syndrome (AHFS) is largely unknown.
Aim: To investigate the prescription characteristics, efficacy and safety of spironolactone in real-world patients with AHFS.
Methods: 5,136 AHFS patients who survived to hospital discharge using a nationwide prospective registry in Korea were analyzed. The primary efficacy outcome was 3-year all-cause mortality.
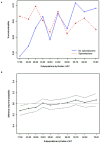
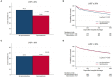
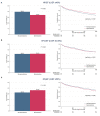
Results: Spironolactone was prescribed in 2,402 (46.8%) at discharge: <25 mg in 890 patients (37.1%), ≥25 mg, and <50 mg in 1,154 patients (48.0%), and ≥50 mg in 358 patients (14.9%). Patients treated with spironolactone had a lower proportion of chronic renal failure and renal replacement therapy during hospitalization and had lower serum creatinine level than those who did not. In overall patients, 3-year mortality was not different in both groups (35.9 vs. 34.5%, P = 0.279). The incidence of renal injury and hyperkalemia was 2.2% and 4.3%, respectively, at the first follow-up visit. The treatment effect of spironolactone on mortality was different across subpopulations according to LVEF. The use of spironolactone was associated with a significant reduction in 3-year morality in patients with LVEF ≤ 26% (33.8 vs. 44.3%, P < 0.001; adjusted HR 0.79, 95% CI 0.64-0.97, P = 0.023), but not in patients with LVEF > 26%.
Conclusions: Although spironolactone was frequently used at lower doses in real-world practice, use of spironolactone significantly reduced 3-year mortality in patients with severely reduced LVEF with acceptable safety profile. However, our findings remain prone to various biases and further prospective randomized controlled studies are needed to confirm these findings.
Keywords: acute heart failure syndrome; drug therapy; mineralocorticoid receptor antagonists; outcome; spironolactone.
Copyright © 2022 Na, Youn, Lee, Jeon, Lee, Cho, Choi, Jeon, Lee, Kim, Kim, Hwang, Cho, Chae, Kang, Choi, Yoo, Kim, Oh and Baek.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Spironolactone and Outcomes in Older Patients with Heart Failure and Reduced Ejection Fraction.Am J Med. 2019 Jan;132(1):71-80.e1. doi: 10.1016/j.amjmed.2018.09.011. Epub 2018 Sep 19. Am J Med. 2019. PMID: 30240686 Free PMC article.
-
Effectiveness and safety of early treatment with spironolactone for new-onset acute heart failure.J Hosp Med. 2024 Apr;19(4):267-277. doi: 10.1002/jhm.13317. Epub 2024 Feb 28. J Hosp Med. 2024. PMID: 38415888
-
Racial Differences in Characteristics and Outcomes of Patients With Heart Failure and Preserved Ejection Fraction in the Treatment of Preserved Cardiac Function Heart Failure Trial.Circ Heart Fail. 2018 Mar;11(3):e004457. doi: 10.1161/CIRCHEARTFAILURE.117.004457. Circ Heart Fail. 2018. PMID: 29664406
-
Mineralocorticoid receptor antagonists in heart failure with preserved ejection fraction (HFpEF).Int J Cardiol. 2015 Dec 1;200:15-9. doi: 10.1016/j.ijcard.2015.07.038. Int J Cardiol. 2015. PMID: 26404747 Review.
-
Safety profile of mineralocorticoid receptor antagonists: Spironolactone and eplerenone.Int J Cardiol. 2015 Dec 1;200:25-9. doi: 10.1016/j.ijcard.2015.05.127. Epub 2015 May 21. Int J Cardiol. 2015. PMID: 26404748 Review.
Cited by
-
Korean Society of Heart Failure Guidelines for the Management of Heart Failure: Treatment.Korean Circ J. 2023 Apr;53(4):217-238. doi: 10.4070/kcj.2023.0047. Korean Circ J. 2023. PMID: 37161681 Free PMC article. Review.
-
Korean Society of Heart Failure Guidelines for the Management of Heart Failure: Treatment.Int J Heart Fail. 2023 Apr 10;5(2):66-81. doi: 10.36628/ijhf.2023.0011. eCollection 2023 Apr. Int J Heart Fail. 2023. PMID: 37180564 Free PMC article. Review.
References
-
- Sztechman D, Czarzasta K, Cudnoch-Jedrzejewska A, Szczepanska-Sadowska E, Zera T. Aldosterone and mineralocorticoid receptors in regulation of the cardiovascular system and pathological remodelling of the heart and arteries. J Physiol Pharmacol. (2018) 69:829–45. 10.26402/jpp.2018.6.01 - DOI - PubMed
-
- Maddox TM, Januzzi JL Jr, Allen LA, Breathett K, Butler J, Davis LL, et al. . 2021 Update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the american college of cardiology solution set oversight committee. J Am Coll Cardiol. (2021) 77:772–810. 10.1016/j.jacc.2020.11.022 - DOI - PubMed
-
- FDA . FDA Briefing document cardiovascular and renal drugs advisory committee meeting december 16, 2020: spironolactone for heart failure with preserved ejection fraction (HFpEF). Available online at: https://www.fda.gov/media/144411/download (accessed December 16, 2020).
LinkOut - more resources
Full Text Sources
Medical

