Chagas Immunochromatographic Rapid Test in the Serological Diagnosis of Trypanosoma cruzi Infection in Wild and Domestic Canids
- PMID: 35273924
- PMCID: PMC8902141
- DOI: 10.3389/fcimb.2022.835383
Chagas Immunochromatographic Rapid Test in the Serological Diagnosis of Trypanosoma cruzi Infection in Wild and Domestic Canids
Abstract
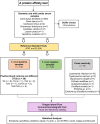
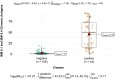
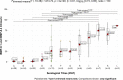
Canis lupus familiaris (domestic dog) represents a reliable sentinel for the occurrence of a well-established transmission cycle of Trypanosoma cruzi among wild mammals in the surroundings and, consequently, where the risk of human infection exists. Serological diagnosis is the chosen method to identify T. cruzi infection in dogs that, in Brazil, rarely present positive parasitological tests. The use of recombinant chimeric parasitic antigens results in a sensitive and specific serological diagnostic test in contrast to the use of crude T. cruzi antigens. Our objective was to evaluate the Chagas/Bio-Manguinhos Lateral Flow Immunochromatographic Rapid Test (Chagas-LFRT) for the diagnosis of T. cruzi infection in domestic dogs and the potential of application of this diagnostic platform to wild canid species. Two recombinant proteins (IBMP-8.1 and IBMP-8.4) that displayed the best performance in the enzyme immunoassay (ELISA) in previous studies were tested in a platform with two diagnostic bands. A panel of 281 dog serum samples was evaluated: 133 positive for T. cruzi by serological diagnosis, including 20 samples with positive blood cultures belonging to different discrete typing units (DTUs); 129 negative samples; and 19 samples from dogs infected by other trypanosomatids: Leishmania infantum, Trypanosoma rangeli, Trypanosoma caninum and Crithidia mellificae, in addition to samples infected by Anaplasma platys, Dirofilaria immitis and Erlichia sp. that were employed to evaluate eventual cross-reactions. We also evaluated the Chagas-LFRT to detect T. cruzi infection in 9 serum samples from six wild canid species. We observed that the intensity pattern of the bands was directly proportional to the serological titer observed in IFAT. The sensitivity was 94%, the specificity was 91% according to the ROC curve, and the defined cutoff was an optical density of 4.8. The agreement obtained was considered substantial by the kappa analysis (84%). From T. cruzi positive hemoculture samples, 88.9% were positive by Chagas-LFRT. The test was efficient in recognizing infections by five of the six T. cruzi DTUs. Cross-reactions were not observed in infections by L. infantum, T. rangeli, T. caninum and D. immitis; however, they were observed in sera of dogs infected by Crithidia mellificae, Anaplasma sp. and Erlichia sp. A strong reaction was observed when serum samples from wild canids were submitted to the Protein A affinity test, confirming its applicability for these species. This test will allow rapid preventive actions in areas with high risk to the emergence of Chagas disease in a safer, reliable, low-cost and immediate manner, without the need for more complex laboratory tests.
Keywords: Trypanosoma cruzi; dead-end and sentinel hosts; domestic and wild canids; quimeric antigens; rapid test.
Copyright © 2022 Rodrigues, Santos, Silva, Barros, Bernardo, Diniz, Rubim, Roque, Jansen, Silva and Xavier.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Technological advances in the serological diagnosis of Chagas disease in dogs and cats: a systematic review.Parasit Vectors. 2022 Sep 27;15(1):343. doi: 10.1186/s13071-022-05476-4. Parasit Vectors. 2022. PMID: 36167575 Free PMC article. Review.
-
Chagas Disease: A Silent Threat for Dogs and Humans.Int J Mol Sci. 2024 Mar 29;25(7):3840. doi: 10.3390/ijms25073840. Int J Mol Sci. 2024. PMID: 38612650 Free PMC article. Review.
-
Trypanosoma Species in Small Nonflying Mammals in an Area With a Single Previous Chagas Disease Case.Front Cell Infect Microbiol. 2022 Feb 11;12:812708. doi: 10.3389/fcimb.2022.812708. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35223545 Free PMC article.
-
Performance of recombinant chimeric proteins in the serological diagnosis of Trypanosoma cruzi infection in dogs.PLoS Negl Trop Dis. 2019 Jun 26;13(6):e0007545. doi: 10.1371/journal.pntd.0007545. eCollection 2019 Jun. PLoS Negl Trop Dis. 2019. PMID: 31242195 Free PMC article.
-
Seroprevalence and detection of Trypanosoma cruzi in dogs living in a non-endemic area for Chagas disease in the legal Amazon region, Brazil.Vet Parasitol Reg Stud Reports. 2021 Dec;26:100648. doi: 10.1016/j.vprsr.2021.100648. Epub 2021 Sep 27. Vet Parasitol Reg Stud Reports. 2021. PMID: 34879958
Cited by
-
Technological advances in the serological diagnosis of Chagas disease in dogs and cats: a systematic review.Parasit Vectors. 2022 Sep 27;15(1):343. doi: 10.1186/s13071-022-05476-4. Parasit Vectors. 2022. PMID: 36167575 Free PMC article. Review.
-
Chagas Disease: A Silent Threat for Dogs and Humans.Int J Mol Sci. 2024 Mar 29;25(7):3840. doi: 10.3390/ijms25073840. Int J Mol Sci. 2024. PMID: 38612650 Free PMC article. Review.
-
Leishmania infantum infecting the carnivore Nasua nasua from urban forest fragments in an endemic area of visceral leishmaniasis in Brazilian Midwest.Front Cell Infect Microbiol. 2023 Jan 13;12:1050339. doi: 10.3389/fcimb.2022.1050339. eCollection 2022. Front Cell Infect Microbiol. 2023. PMID: 36710973 Free PMC article.
-
Laboratory evaluation of eleven rapid diagnostic tests for serological diagnosis of Chagas disease in Colombia.PLoS Negl Trop Dis. 2023 Aug 22;17(8):e0011547. doi: 10.1371/journal.pntd.0011547. eCollection 2023 Aug. PLoS Negl Trop Dis. 2023. PMID: 37607214 Free PMC article.
-
Evaluation of chimeric recombinant antigens for the serodiagnosis of Trypanosoma cruzi in dogs: a promising tool for Chagas disease surveillance.Parasit Vectors. 2024 Jul 15;17(1):305. doi: 10.1186/s13071-024-06376-5. Parasit Vectors. 2024. PMID: 39010122 Free PMC article.
References
-
- Bjorck L., KronvalI G. (1984). Purification and Some Properties of Streptococcal Protein G, a Novel IgG-Binding Reagent. J. lmmunol. 133, 969–974. - PubMed
-
- Brandão E. M. V., Xavier S. C. C., Rocha F. L., Lima C. F. M., Candeias Í.Z., Lemos F. G., et al. . (2020). Wild and Domestic Canids and Their Interactions in the Transmission Cycles of Trypanosoma Cruzi and Leishmania Spp. In an Area of the Brazilian Cerrado. Pathogens 9 (10), 818. doi: 10.3390/pathogens9100818 - DOI - PMC - PubMed
-
- Camussone C., Gonzalez V., Belluzo M. S., Pujato N., Ribone M. E., Lagier C. M., et al. . (2009). Comparison of Recombinant Trypanosoma Cruzi Peptide Mixtures Versus Multiepitope Chimeric Proteins as Sensitizing Antigens for Immunodiagnosis. Clin. Vaccine Immunol.: CVI 16, 899–905. doi: 10.1128/CVI.00005-09 - DOI - PMC - PubMed
-
- Cohen J. (1960). A Coefficient of Agreement for Nominal Scales. Educational and Psychological Measurement 20 (1), 37–46. doi: 10.1177/001316446002000104 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

