Chromosome Segregation in the Oocyte: What Goes Wrong during Aging
- PMID: 35270022
- PMCID: PMC8911062
- DOI: 10.3390/ijms23052880
Chromosome Segregation in the Oocyte: What Goes Wrong during Aging
Abstract
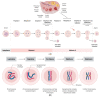
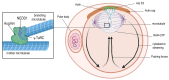
Human female fertility and reproductive lifespan decrease significantly with age, resulting in an extended post-reproductive period. The central dogma in human female reproduction contains two important aspects. One is the pool of oocytes in the human ovary (the ovarian reserve; approximately 106 at birth), which diminishes throughout life until menopause around the age of 50 (approximately 103 oocytes) in women. The second is the quality of oocytes, including the correctness of meiotic divisions, among other factors. Notably, the increased rate of sub- and infertility, aneuploidy, miscarriages, and birth defects are associated with advanced maternal age, especially in women above 35 years of age. This postponement is also relevant for human evolution; decades ago, the female aging-related fertility drop was not as important as it is today because women were having their children at a younger age. Spindle assembly is crucial for chromosome segregation during each cell division and oocyte maturation, making it an important event for euploidy. Consequently, aberrations in this segregation process, especially during the first meiotic division in human eggs, can lead to implantation failure or spontaneous abortion. Today, human reproductive medicine is also facing a high prevalence of aneuploidy, even in young females. However, the shift in the reproductive phase of humans and the strong increase in errors make the problem much more dramatic at later stages of the female reproductive phase. Aneuploidy in human eggs could be the result of the non-disjunction of entire chromosomes or sister chromatids during oocyte meiosis, but partial or segmental aneuploidies are also relevant. In this review, we intend to describe the relevance of the spindle apparatus during oocyte maturation for proper chromosome segregation in the context of maternal aging and the female reproductive lifespan.
Keywords: aneuploidy; chromosome segregation; euploidy; maternal aging; oocytes; spindle assembly; spindle formation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Aneuploidy in human eggs: contributions of the meiotic spindle.Biochem Soc Trans. 2021 Feb 26;49(1):107-118. doi: 10.1042/BST20200043. Biochem Soc Trans. 2021. PMID: 33449109 Free PMC article. Review.
-
Age-dependent integrity of the meiotic spindle assembly checkpoint in females requires Aurora kinase B.Aging Cell. 2021 Nov;20(11):e13489. doi: 10.1111/acel.13489. Epub 2021 Oct 26. Aging Cell. 2021. PMID: 34704342 Free PMC article.
-
Mechanisms of oocyte aneuploidy associated with advanced maternal age.Mutat Res Rev Mutat Res. 2020 Jul-Sep;785:108320. doi: 10.1016/j.mrrev.2020.108320. Epub 2020 Jul 4. Mutat Res Rev Mutat Res. 2020. PMID: 32800274 Review.
-
Human female meiosis revised: new insights into the mechanisms of chromosome segregation and aneuploidies from advanced genomics and time-lapse imaging.Hum Reprod Update. 2017 Nov 1;23(6):706-722. doi: 10.1093/humupd/dmx026. Hum Reprod Update. 2017. PMID: 28961822 Review.
-
Activin Decoy Receptor ActRIIB:Fc Lowers FSH and Therapeutically Restores Oocyte Yield, Prevents Oocyte Chromosome Misalignments and Spindle Aberrations, and Increases Fertility in Midlife Female SAMP8 Mice.Endocrinology. 2016 Mar;157(3):1234-47. doi: 10.1210/en.2015-1702. Epub 2015 Dec 29. Endocrinology. 2016. PMID: 26713784 Free PMC article.
Cited by
-
Analysis of the Potential Relationship between Aging and Pulmonary Fibrosis Based on Transcriptome.Life (Basel). 2022 Nov 23;12(12):1961. doi: 10.3390/life12121961. Life (Basel). 2022. PMID: 36556326 Free PMC article.
-
Healthy Live Births after the Transfer of Mosaic Embryos: Self-Correction or PGT-A Overestimation?Genes (Basel). 2023 Dec 21;15(1):18. doi: 10.3390/genes15010018. Genes (Basel). 2023. PMID: 38275600 Free PMC article. Review.
-
Cytokine-Supplemented Maturation Medium Enhances Cytoplasmic and Nuclear Maturation in Bovine Oocytes.Animals (Basel). 2024 Jun 20;14(12):1837. doi: 10.3390/ani14121837. Animals (Basel). 2024. PMID: 38929455 Free PMC article.
-
Polyamine metabolite spermidine rejuvenates oocyte quality by enhancing mitophagy during female reproductive aging.Nat Aging. 2023 Nov;3(11):1372-1386. doi: 10.1038/s43587-023-00498-8. Epub 2023 Oct 16. Nat Aging. 2023. PMID: 37845508
-
Oocyte Aging: A Multifactorial Phenomenon in A Unique Cell.Aging Dis. 2024 Feb 1;15(1):5-21. doi: 10.14336/AD.2023.0527. Aging Dis. 2024. PMID: 37307833 Free PMC article. Review.
References
-
- Ely D.M., Hamilton B.E. Trends in Fertility and Mother’s Age at First Birth Among Rural and Metropolitan Counties: United States, 2007–2017. National Center for Health Statistics; Hyattsville, MD, USA: 2018. pp. 1–8. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

