Human Amnion Epithelial Cells: A Potential Cell Source for Pulp Regeneration?
- PMID: 35269973
- PMCID: PMC8911206
- DOI: 10.3390/ijms23052830
Human Amnion Epithelial Cells: A Potential Cell Source for Pulp Regeneration?
Abstract
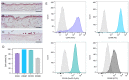
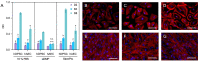
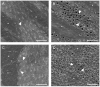
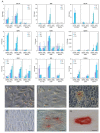
The aim of this study was to analyze the suitability of pluripotent stem cells derived from the amnion (hAECs) as a potential cell source for revitalization in vitro. hAECs were isolated from human placentas, and dental pulp stem cells (hDPSCs) and dentin matrix proteins (eDMPs) were obtained from human teeth. Both hAECs and hDPSCs were cultured with 10% FBS, eDMPs and an osteogenic differentiation medium (StemPro). Viability was assessed by MTT and cell adherence to dentin was evaluated by scanning electron microscopy. Furthermore, the expression of mineralization-, odontogenic differentiation- and epithelial-mesenchymal transition-associated genes was analyzed by quantitative real-time PCR, and mineralization was evaluated through Alizarin Red staining. The viability of hAECs was significantly lower compared with hDPSCs in all groups and at all time points. Both hAECs and hDPSCs adhered to dentin and were homogeneously distributed. The regulation of odontoblast differentiation- and mineralization-associated genes showed the lack of transition of hAECs into an odontoblastic phenotype; however, genes associated with epithelial-mesenchymal transition were significantly upregulated in hAECs. hAECs showed small amounts of calcium deposition after osteogenic differentiation with StemPro. Pluripotent hAECs adhere on dentin and possess the capacity to mineralize. However, they presented an unfavorable proliferation behavior and failed to undergo odontoblastic transition.
Keywords: dental pulp stem cells; dentin matrix proteins; human amnion epithelial cells; odontoblastic differentiation; revitalization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
BACH1 regulates the proliferation and odontoblastic differentiation of human dental pulp stem cells.BMC Oral Health. 2022 Nov 24;22(1):536. doi: 10.1186/s12903-022-02588-2. BMC Oral Health. 2022. PMID: 36424585 Free PMC article.
-
Promotive Effect of FBXO32 on the Odontoblastic Differentiation of Human Dental Pulp Stem Cells.Int J Mol Sci. 2023 Apr 22;24(9):7708. doi: 10.3390/ijms24097708. Int J Mol Sci. 2023. PMID: 37175415 Free PMC article.
-
C5L2 Regulates DMP1 Expression during Odontoblastic Differentiation.J Dent Res. 2019 May;98(5):597-604. doi: 10.1177/0022034518820461. Epub 2019 Jan 31. J Dent Res. 2019. PMID: 30702959 Free PMC article.
-
A Survey and Critical Evaluation of Isolation, Culture, and Cryopreservation Methods of Human Amniotic Epithelial Cells.Cell Cycle. 2022 Apr;21(7):655-673. doi: 10.1080/15384101.2021.2020015. Epub 2022 Mar 15. Cell Cycle. 2022. PMID: 35289707 Free PMC article. Review.
-
[Advances on human amniotic epithelial cells and its clinical application potential].Sheng Li Xue Bao. 2022 Feb 25;74(1):80-92. Sheng Li Xue Bao. 2022. PMID: 35199129 Review. Chinese.
Cited by
-
Engineering the Future of Dental Health: Exploring Molecular Advancements in Dental Pulp Regeneration.Int J Mol Sci. 2023 Jul 14;24(14):11453. doi: 10.3390/ijms241411453. Int J Mol Sci. 2023. PMID: 37511210 Free PMC article.
-
A Compilation of Study Models for Dental Pulp Regeneration.Int J Mol Sci. 2022 Nov 18;23(22):14361. doi: 10.3390/ijms232214361. Int J Mol Sci. 2022. PMID: 36430838 Free PMC article. Review.

