Treatment of vascular graft infections: gentamicin-coated ePTFE grafts reveals strong antibacterial properties in vitro
- PMID: 35267117
- PMCID: PMC8913444
- DOI: 10.1007/s10856-022-06650-x
Treatment of vascular graft infections: gentamicin-coated ePTFE grafts reveals strong antibacterial properties in vitro
Abstract
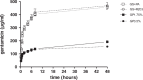
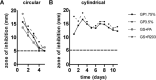
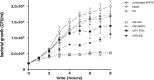
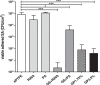
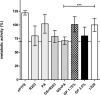
Vascular graft infections (VGI) are severe complications in prosthetic vascular surgery with an incidence ranging from 1 to 6%. In these cases, synthetic grafts are commonly used in combination with antimicrobial agents. Expanded polytetrafluoroethylene (ePTFE) is in clinical use as a synthetic graft material and shows promising results by influencing bacterial adhesion. However, the literature on antibiotic-bound ePTFE grafts is scarce. Gentamicin is a frequently used antibiotic for local treatment of surgical site infections, but has not been evaluated as antimicrobial agent on ePTFE grafts. In this study, we examine the antimicrobial efficacy and biocompatibility of novel types of gentamicin-coated ePTFE grafts in vitro. ePTFE grafts coated with gentamicin salt formulations with covalently-bound palmitate were evaluated in two drug concentrations (GP1.75% and GP3.5%). To investigate effects from types of formulations, also suspensions of gentamicin in palmitate as well as polylactide were used at comparable levels (GS + PA and GS + R203). Antibacterial efficacies were estimated by employing a zone of inhibition, growth inhibition and bacterial adhesion assay against Staphylococcus aureus (SA). Cytotoxicity was determined with murine fibroblasts according to the ISO standard 10993-5. Gentamicin-coated ePTFE grafts show low bacterial adherence and strong antibacterial properties in vitro against SA. Bactericidal inhibition lasted until day 11. Highest biocompatibility was achieved using gentamicin palmitate GP1.75% coated ePTFE grafts. ePTFE grafts with gentamicin-coating are effective in vitro against SA growth and adherence. Most promising results regarding antimicrobial properties and biocompatibility were shown with chemically bounded gentamicin palmitate GP1.75% coatings. Graphical abstract.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Antibiotic-coated ePTFE decreases graft colonization and neointimal hyperplasia.J Surg Res. 2009 Oct;156(2):199-204. doi: 10.1016/j.jss.2009.01.016. Epub 2009 Feb 12. J Surg Res. 2009. PMID: 19481768
-
Rapamycin-coated expanded polytetrafluoroethylene bypass grafts exhibit decreased anastomotic neointimal hyperplasia in a porcine model.J Vasc Surg. 2005 Nov;42(5):980-8. doi: 10.1016/j.jvs.2005.06.018. J Vasc Surg. 2005. PMID: 16275457
-
Silyl-heparin adsorption improves the in vivo thromboresistance of carbon-coated polytetrafluoroethylene vascular grafts.Am J Surg. 2003 Nov;186(5):556-60. doi: 10.1016/j.amjsurg.2003.07.015. Am J Surg. 2003. PMID: 14599625
-
ePTFE-based biomedical devices: An overview of surgical efficiency.J Biomed Mater Res B Appl Biomater. 2022 Feb;110(2):302-320. doi: 10.1002/jbm.b.34928. Epub 2021 Sep 14. J Biomed Mater Res B Appl Biomater. 2022. PMID: 34520627 Review.
-
Calcification of Synthetic Vascular Grafts: A Systematic Review.EJVES Vasc Forum. 2023 May 29;60:1-7. doi: 10.1016/j.ejvsvf.2023.05.013. eCollection 2023. EJVES Vasc Forum. 2023. PMID: 37416860 Free PMC article. Review.
References
-
- Seeger JM. Management of patients with prosthetic vascular graft infection. Am Surg. 2000;66(2):166–77. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

