Aβ oligomers trigger necroptosis-mediated neurodegeneration via microglia activation in Alzheimer's disease
- PMID: 35264247
- PMCID: PMC8908658
- DOI: 10.1186/s40478-022-01332-9
Aβ oligomers trigger necroptosis-mediated neurodegeneration via microglia activation in Alzheimer's disease
Abstract
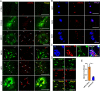
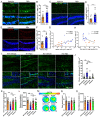
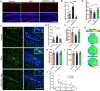
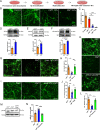
Alzheimer's disease (AD) is a major adult-onset neurodegenerative condition with no available treatment. Compelling reports point amyloid-β (Aβ) as the main etiologic agent that triggers AD. Although there is extensive evidence of detrimental crosstalk between Aβ and microglia that contributes to neuroinflammation in AD, the exact mechanism leading to neuron death remains unknown. Using postmortem human AD brain tissue, we show that Aβ pathology is associated with the necroptosis effector pMLKL. Moreover, we found that the burden of Aβ oligomers (Aβo) correlates with the expression of key markers of necroptosis activation. Additionally, inhibition of necroptosis by pharmacological or genetic means, reduce neurodegeneration and memory impairment triggered by Aβo in mice. Since microglial activation is emerging as a central driver for AD pathogenesis, we then tested the contribution of microglia to the mechanism of Aβo-mediated necroptosis activation in neurons. Using an in vitro model, we show that conditioned medium from Aβo-stimulated microglia elicited necroptosis in neurons through activation of TNF-α signaling, triggering extensive neurodegeneration. Notably, necroptosis inhibition provided significant neuronal protection. Together, these findings suggest that Aβo-mediated microglia stimulation in AD contributes to necroptosis activation in neurons and neurodegeneration. As necroptosis is a druggable degenerative mechanism, our findings might have important therapeutic implications to prevent the progression of AD.
Keywords: Alzheimer’s disease; Amyloid-β oligomers; Microglia; Necroptosis; Neurodegeneration; Neuroprotection.
© 2022. The Author(s).
Conflict of interest statement
The authors report no competing interests in relation to this manuscript.
Figures

Similar articles
-
Injection of exogenous amyloid-β oligomers aggravated cognitive deficits, and activated necroptosis, in APP23 transgenic mice.Brain Res. 2023 Dec 15;1821:148565. doi: 10.1016/j.brainres.2023.148565. Epub 2023 Sep 6. Brain Res. 2023. PMID: 37683777
-
Donepezil inhibits the amyloid-beta oligomer-induced microglial activation in vitro and in vivo.Neurotoxicology. 2014 Jan;40:23-32. doi: 10.1016/j.neuro.2013.10.004. Epub 2013 Nov 1. Neurotoxicology. 2014. PMID: 24189446
-
Fibrillar Aβ triggers microglial proteome alterations and dysfunction in Alzheimer mouse models.Elife. 2020 Jun 8;9:e54083. doi: 10.7554/eLife.54083. Elife. 2020. PMID: 32510331 Free PMC article.
-
Necroptosis and Alzheimer's Disease: Pathogenic Mechanisms and Therapeutic Opportunities.J Alzheimers Dis. 2023;94(s1):S367-S386. doi: 10.3233/JAD-220809. J Alzheimers Dis. 2023. PMID: 36463451 Free PMC article. Review.
-
Amyloid β oligomers in Alzheimer's disease pathogenesis, treatment, and diagnosis.Acta Neuropathol. 2015 Feb;129(2):183-206. doi: 10.1007/s00401-015-1386-3. Epub 2015 Jan 22. Acta Neuropathol. 2015. PMID: 25604547 Free PMC article. Review.
Cited by
-
Microglia either promote or restrain TRAIL-mediated excitotoxicity caused by Aβ1-42 oligomers.J Neuroinflammation. 2024 Sep 1;21(1):215. doi: 10.1186/s12974-024-03208-2. J Neuroinflammation. 2024. PMID: 39218898 Free PMC article.
-
Uncovering the Achilles heel of genetic heterogeneity: machine learning-based classification and immunological properties of necroptosis clusters in Alzheimer's disease.Front Aging Neurosci. 2023 Sep 20;15:1249682. doi: 10.3389/fnagi.2023.1249682. eCollection 2023. Front Aging Neurosci. 2023. PMID: 37799623 Free PMC article.
-
Neuronal cell death mechanisms in Alzheimer's disease: An insight.Front Mol Neurosci. 2022 Aug 25;15:937133. doi: 10.3389/fnmol.2022.937133. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36090249 Free PMC article. Review.
-
The role of ZBP1 in eccentric exercise-induced skeletal muscle necroptosis.J Muscle Res Cell Motil. 2023 Dec;44(4):311-323. doi: 10.1007/s10974-023-09660-6. Epub 2023 Oct 27. J Muscle Res Cell Motil. 2023. PMID: 37889396
-
Necroptosis in CNS diseases: Focus on astrocytes.Front Aging Neurosci. 2023 Jan 27;14:1016053. doi: 10.3389/fnagi.2022.1016053. eCollection 2022. Front Aging Neurosci. 2023. PMID: 36778591 Free PMC article. Review.
References
-
- Alzheimer’s Association, 2020 Alzheimer’s disease facts and figures. Alzheimer’s Dementia16, 391–460 (2020).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

