Oocyte activation deficiency and assisted oocyte activation: mechanisms, obstacles and prospects for clinical application
- PMID: 35261925
- PMCID: PMC8894871
- DOI: 10.1093/hropen/hoac003
Oocyte activation deficiency and assisted oocyte activation: mechanisms, obstacles and prospects for clinical application
Abstract
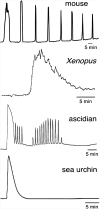
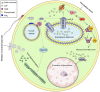
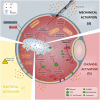
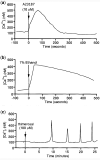
Background: Oocyte activation deficiency (OAD) is attributed to the majority of cases underlying failure of ICSI cycles, the standard treatment for male factor infertility. Oocyte activation encompasses a series of concerted events, triggered by sperm-specific phospholipase C zeta (PLCζ), which elicits increases in free cytoplasmic calcium (Ca2+) in spatially and temporally specific oscillations. Defects in this specific pattern of Ca2+ release are directly attributable to most cases of OAD. Ca2+ release can be clinically mediated via assisted oocyte activation (AOA), a combination of mechanical, electrical and/or chemical stimuli which artificially promote an increase in the levels of intra-cytoplasmic Ca2+. However, concerns regarding safety and efficacy underlie potential risks that must be addressed before such methods can be safely widely used.
Objective and rationale: Recent advances in current AOA techniques warrant a review of the safety and efficacy of these practices, to determine the extent to which AOA may be implemented in the clinic. Importantly, the primary challenges to obtaining data on the safety and efficacy of AOA must be determined. Such questions require urgent attention before widespread clinical utilization of such protocols can be advocated.
Search methods: A literature review was performed using databases including PubMed, Web of Science, Medline, etc. using AOA, OAD, calcium ionophores, ICSI, PLCζ, oocyte activation, failed fertilization and fertilization failure as keywords. Relevant articles published until June 2019 were analysed and included in the review, with an emphasis on studies assessing large-scale efficacy and safety.
Outcomes: Contradictory studies on the safety and efficacy of AOA do not yet allow for the establishment of AOA as standard practice in the clinic. Heterogeneity in study methodology, inconsistent sample inclusion criteria, non-standardized outcome assessments, restricted sample size and animal model limitations render AOA strictly experimental. The main scientific concern impeding AOA utilization in the clinic is the non-physiological method of Ca2+ release mediated by most AOA agents, coupled with a lack of holistic understanding regarding the physiological mechanism(s) underlying Ca2+ release at oocyte activation.
Limitations reasons for caution: The number of studies with clinical relevance using AOA remains significantly low. A much wider range of studies examining outcomes using multiple AOA agents are required.
Wider implications: In addition to addressing the five main challenges of studies assessing AOA safety and efficacy, more standardized, large-scale, multi-centre studies of AOA, as well as long-term follow-up studies of children born from AOA, would provide evidence for establishing AOA as a treatment for infertility. The delivery of an activating agent that can more accurately recapitulate physiological fertilization, such as recombinant PLCζ, is a promising prospect for the future of AOA. Further to PLCζ, many other avenues of physiological oocyte activation also require urgent investigation to assess other potential physiological avenues of AOA.
Study funding/competing interests: D.G. was supported by Stanford University's Bing Overseas Study Program. J.K. was supported by a Healthcare Research Fellowship Award (HF-14-16) made by Health and Care Research Wales (HCRW), alongside a National Science, Technology, and Innovation plan (NSTIP) project grant (15-MED4186-20) awarded by the King Abdulaziz City for Science and Technology (KACST). The authors have no competing interests to declare.
Keywords: ICSI; assisted oocyte activation (AOA); calcium; calcium ionophores; male infertility; oocyte; oocyte activation; oocyte activation deficiency (OAD); phospholipase C zeta (PLCζ); sperm.
© The Author(s) 2022. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Figures
Similar articles
-
The mammalian sperm factor phospholipase C zeta is critical for early embryo division and pregnancy in humans and mice.Hum Reprod. 2024 Jun 3;39(6):1256-1274. doi: 10.1093/humrep/deae078. Hum Reprod. 2024. PMID: 38670547 Free PMC article.
-
Total fertilization failure after ICSI: insights into pathophysiology, diagnosis, and management through artificial oocyte activation.Hum Reprod Update. 2023 Jul 5;29(4):369-394. doi: 10.1093/humupd/dmad007. Hum Reprod Update. 2023. PMID: 36977357 Review.
-
Antigen unmasking enhances visualization efficacy of the oocyte activation factor, phospholipase C zeta, in mammalian sperm.Mol Hum Reprod. 2017 Jan;23(1):54-67. doi: 10.1093/molehr/gaw073. Epub 2016 Dec 8. Mol Hum Reprod. 2017. PMID: 27932551
-
Artificial oocyte activation to improve reproductive outcomes in women with previous fertilization failure: a systematic review and meta-analysis of RCTs.Hum Reprod. 2015 Aug;30(8):1831-41. doi: 10.1093/humrep/dev136. Epub 2015 Jun 16. Hum Reprod. 2015. PMID: 26082476 Review.
-
High rate of detected variants in male PLCZ1 and ACTL7A genes causing failed fertilization after ICSI.Hum Reprod Open. 2024 Sep 28;2024(4):hoae057. doi: 10.1093/hropen/hoae057. eCollection 2024. Hum Reprod Open. 2024. PMID: 39411542 Free PMC article.
Cited by
-
Applications of advances in mRNA-based platforms as therapeutics and diagnostics in reproductive technologies.Front Cell Dev Biol. 2023 May 26;11:1198848. doi: 10.3389/fcell.2023.1198848. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37305677 Free PMC article. Review.
-
Septins as key players in spermatogenesis, fertilisation and pre-implantation embryogenic cytoplasmic dynamics.Cell Commun Signal. 2024 Oct 28;22(1):523. doi: 10.1186/s12964-024-01889-z. Cell Commun Signal. 2024. PMID: 39468561 Free PMC article. Review.
-
Early rescue oocyte activation at 5 h post-ICSI is a useful strategy for avoiding unexpected fertilization failure and low fertilization in ICSI cycles.Front Endocrinol (Lausanne). 2024 Jan 4;14:1301505. doi: 10.3389/fendo.2023.1301505. eCollection 2023. Front Endocrinol (Lausanne). 2024. PMID: 38239979 Free PMC article.
-
ART constraints imposed by the complexities of oocyte activation.J Assist Reprod Genet. 2022 Jun;39(6):1217-1218. doi: 10.1007/s10815-022-02525-w. J Assist Reprod Genet. 2022. PMID: 35657455 Free PMC article. No abstract available.
-
The Therapeutic and Diagnostic Potential of Phospholipase C Zeta, Oocyte Activation, and Calcium in Treating Human Infertility.Pharmaceuticals (Basel). 2023 Mar 15;16(3):441. doi: 10.3390/ph16030441. Pharmaceuticals (Basel). 2023. PMID: 36986540 Free PMC article. Review.
References
-
- Aarabi M, Balakier H, Bashar S, Moskovtsev SI, Sutovsky P, Librach CL, Oko R. Sperm content of postacrosomal WW binding protein is related to fertilization outcomes in patients undergoing assisted reproductive technology. Fertil Steril 2014a;102:440–447. - PubMed
-
- Aarabi M, Balakier H, Bashar S, Moskovtsev SI, Sutovsky P, Librach CL, Oko R. Sperm-derived WW domain-binding protein, PAWP, elicits calcium oscillations and oocyte activation in humans and mice. FASEB J 2014b;28:4434–4440. - PubMed
-
- Aarabi M, Qin Z, Xu W, Mewburn J, Oko R. Sperm-borne protein, PAWP, initiates zygotic development in Xenopus laevis by eliciting intracellular calcium release. Mol Reprod Dev 2009;77:249–256. - PubMed
-
- Abel K, Healey M, Finch S, Osianlis T, Vollenhoven B. Associations between embryo grading and congenital malformations in IVF/ICSI pregnancies. Reprod Biomed Online 2019;39:981–989. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
