Low dose DMSO treatment induces oligomerization and accelerates aggregation of α-synuclein
- PMID: 35260646
- PMCID: PMC8904838
- DOI: 10.1038/s41598-022-07706-2
Low dose DMSO treatment induces oligomerization and accelerates aggregation of α-synuclein
Abstract
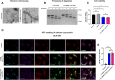
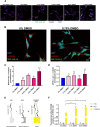
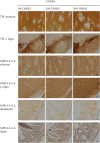
Dimethyl sulfoxide (DMSO) is a highly utilized small molecule that serves many purposes in scientific research. DMSO offers unique polar, aprotic and amphiphilic features, which makes it an ideal solvent for a wide variety of both polar and nonpolar molecules. Furthermore, DMSO is often used as a cryoprotectant in cell-based research. However, recent reports suggest that DMSO, even at low concentration, might interfere with important cellular processes, and cause macromolecular changes to proteins where a shift from α-helical to β-sheet structure can be observed. To investigate how DMSO might influence current research, we assessed biochemical and cellular impacts of DMSO treatment on the structure of the aggregation-prone protein α-synuclein, which plays a central role in the etiology of Parkinson's disease, and other brain-related disorders, collectively termed the synucleinopathies. Here, we found that addition of DMSO increased the particle-size of α-synuclein, and accelerated the formation of seeding-potent fibrils in a dose-dependent manner. These fibrils made in the presence of DMSO were indistinguishable from fibrils made in pure PBS, when assessed by proteolytic digestion, cytotoxic profile and their ability to seed cellular aggregation of α-synuclein. Moreover, as evident through binding to the MJFR-14-6-4-2 antibody, which preferentially recognizes aggregated forms of α-synuclein, and a bimolecular fluorescence complementation assay, cells exposed to DMSO experienced increased aggregation of α-synuclein. However, no observable α-synuclein abnormalities nor differences in neuronal survival were detected after oral DMSO-treatment in either C57BL/6- or α-synuclein transgenic F28 mice. In summary, we demonstrate that low concentrations of DMSO makes α-synuclein susceptible to undergo aggregation both in vitro and in cells. This may affect experimental outcomes when studying α-synuclein in the presence of DMSO, and should call for careful consideration when such experiments are planned.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Tau accelerates α-synuclein aggregation and spreading in Parkinson's disease.Brain. 2022 Oct 21;145(10):3454-3471. doi: 10.1093/brain/awac171. Brain. 2022. PMID: 35552614
-
A glutaminyl cyclase-catalyzed α-synuclein modification identified in human synucleinopathies.Acta Neuropathol. 2021 Sep;142(3):399-421. doi: 10.1007/s00401-021-02349-5. Epub 2021 Jul 26. Acta Neuropathol. 2021. PMID: 34309760 Free PMC article.
-
Quaternary structure of patient-homogenate amplified α-synuclein fibrils modulates seeding of endogenous α-synuclein.Commun Biol. 2022 Sep 30;5(1):1040. doi: 10.1038/s42003-022-03948-y. Commun Biol. 2022. PMID: 36180728 Free PMC article.
-
Neurotoxic conversion of beta-synuclein: a novel approach to generate a transgenic mouse model of synucleinopathies?J Neurol. 2009 Aug;256 Suppl 3:286-92. doi: 10.1007/s00415-009-5246-8. J Neurol. 2009. PMID: 19711118 Review.
-
Anti-aggregation Effects of Phenolic Compounds on α-synuclein.Molecules. 2020 May 24;25(10):2444. doi: 10.3390/molecules25102444. Molecules. 2020. PMID: 32456274 Free PMC article. Review.
Cited by
-
Protein kinase R dependent phosphorylation of α-synuclein regulates its membrane binding and aggregation.PNAS Nexus. 2022 Nov 16;1(5):pgac259. doi: 10.1093/pnasnexus/pgac259. eCollection 2022 Nov. PNAS Nexus. 2022. PMID: 36712380 Free PMC article.
-
The in vitro dynamics of pseudo-vascular network formation.Br J Cancer. 2024 Aug;131(3):457-467. doi: 10.1038/s41416-024-02722-7. Epub 2024 Jun 20. Br J Cancer. 2024. PMID: 38902534 Free PMC article.
-
Monomeric α-synuclein activates the plasma membrane calcium pump.EMBO J. 2023 Dec 1;42(23):e111122. doi: 10.15252/embj.2022111122. Epub 2023 Nov 2. EMBO J. 2023. PMID: 37916890 Free PMC article.
-
Metabolomic and Lipidomic Analysis of Manganese-Associated Parkinsonism: a Case-Control Study in Brescia, Italy.medRxiv [Preprint]. 2024 Sep 6:2024.09.04.24313002. doi: 10.1101/2024.09.04.24313002. medRxiv. 2024. PMID: 39281765 Free PMC article. Preprint.
-
ER stress in mouse serotonin neurons triggers a depressive phenotype alleviated by ketamine targeting eIF2α signaling.iScience. 2024 Apr 18;27(5):109787. doi: 10.1016/j.isci.2024.109787. eCollection 2024 May 17. iScience. 2024. PMID: 38711453 Free PMC article.
References
-
- Rocha EM, De Miranda B, Sanders LH. Alpha-synuclein: Pathology, mitochondrial dysfunction and neuroinflammation in Parkinson’s disease. Neurobiol. Dis. 2018;109:249–257. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

