Alleviating Aspirin-Induced Gastric Injury by Binding Aspirin to β-Lactoglobulin
- PMID: 35256843
- PMCID: PMC8898184
- DOI: 10.2147/DDDT.S351100
Alleviating Aspirin-Induced Gastric Injury by Binding Aspirin to β-Lactoglobulin
Abstract
Purpose: Gastric injury is a major issue for long-term administration of aspirin. In this work, we tried to explore the possibility of using BLG to alleviate aspirin-induced gastric injury, because of excellent abilities of BLG in loading drug molecules.
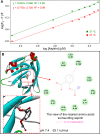
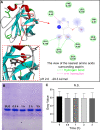
Methods: Various spectroscopic techniques and molecular docking methods were applied to investigate the interaction mechanism between BLG and aspirin. Animal experiments were performed to figure out the effects of taking aspirin-BLG on the stomach.
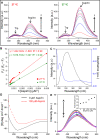
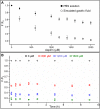
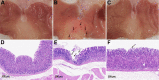
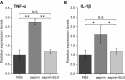
Results: Our results demonstrate that aspirin could bind with BLG to form stable aspirin-BLG complex (the binding constant Kb = 2.051 × 103 M-1). The formation process is endothermic (∆H>0) and the main acting force is hydrophobic force. Our data also show that the aspirin-BLG complex is formed with a higher affinity in simulated gastric fluid and could remain stable for several hours, which might arise from its special binding mode under acidic condition and the resistance of BLG to gastric digestion. Furthermore, animal models (rats with aspirin-induced gastric damage) were built. The results of animal experiments reveal that the oral administration of aspirin-BLG could cause less damage to gastric tissue, and it also hardly triggers obvious inflammatory responses.
Conclusion: This study would contribute to an in-depth understanding of the interaction mechanism between BLG and aspirin. It is reasonable to believe that using BLG to bind with aspirin would be a potential way to alleviate the aspirin-induced gastric injury.
Keywords: aspirin; beta-lactoglobulin; gastric injury; molecular docking; static quenching.
© 2022 Chen et al.
Conflict of interest statement
The authors have no conflicts of interest for this work to declare.
Figures

Similar articles
-
New insights into the binding mechanism between osthole and β-lactoglobulin: Spectroscopic, chemometrics and docking studies.Food Res Int. 2019 Jun;120:226-234. doi: 10.1016/j.foodres.2019.02.042. Epub 2019 Feb 22. Food Res Int. 2019. PMID: 31000234
-
Integrated spectroscopic and computational analyses unravel the molecular interaction of pesticide azinphos-methyl with bovine beta-lactoglobulin.J Mol Recognit. 2024 Jul;37(4):e3086. doi: 10.1002/jmr.3086. Epub 2024 Apr 30. J Mol Recognit. 2024. PMID: 38686702
-
Multi-spectroscopic and molecular docking studies for the pH-dependent interaction of β-lactoglobulin with (-)-epicatechin gallate and/or piceatannol: Influence on antioxidant activity and stability.Spectrochim Acta A Mol Biomol Spectrosc. 2024 May 15;313:124090. doi: 10.1016/j.saa.2024.124090. Epub 2024 Feb 27. Spectrochim Acta A Mol Biomol Spectrosc. 2024. PMID: 38428163
-
Interactions of β-Lactoglobulin with Bovine Submaxillary Mucin vs. Porcine Gastric Mucin: The Role of Hydrophobic and Hydrophilic Residues as Studied by Fluorescence Spectroscopy.Molecules. 2021 Nov 10;26(22):6799. doi: 10.3390/molecules26226799. Molecules. 2021. PMID: 34833889 Free PMC article.
-
Exploring binding properties of naringenin with bovine β-lactoglobulin: a fluorescence, molecular docking and molecular dynamics simulation study.Biophys Chem. 2014 Mar-Apr;187-188:33-42. doi: 10.1016/j.bpc.2014.01.003. Epub 2014 Jan 28. Biophys Chem. 2014. PMID: 24530705
Cited by
-
Acetylsalicylic Acid-Primus Inter Pares in Pharmacology.Molecules. 2022 Dec 1;27(23):8412. doi: 10.3390/molecules27238412. Molecules. 2022. PMID: 36500502 Free PMC article. Review.
References
-
- Schrör K, Voelker M. NSAIDS and Aspirin: Recent Advances and Implications for Clinical Management. Lanas A ed. Springer International Publishing; 2016.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

