Confined migration promotes cancer metastasis through resistance to anoikis and increased invasiveness
- PMID: 35256052
- PMCID: PMC8903834
- DOI: 10.7554/eLife.73150
Confined migration promotes cancer metastasis through resistance to anoikis and increased invasiveness
Abstract
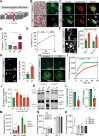
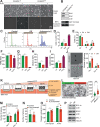
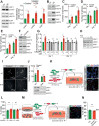
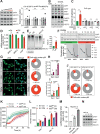
Mechanical stress is known to fuel several hallmarks of cancer, ranging from genome instability to uncontrolled proliferation or invasion. Cancer cells are constantly challenged by mechanical stresses not only in the primary tumour but also during metastasis. However, this latter has seldom been studied with regards to mechanobiology, in particular resistance to anoikis, a cell death programme triggered by loss of cell adhesion. Here, we show in vitro that migrating breast cancer cells develop resistance to anoikis following their passage through microporous membranes mimicking confined migration (CM), a mechanical constriction that cancer cells encounter during metastasis. This CM-induced resistance was mediated by Inhibitory of Apoptosis Proteins, and sensitivity to anoikis could be restored after their inhibition using second mitochondria-derived activator of caspase (SMAC) mimetics. Anoikis-resistant mechanically stressed cancer cells displayed enhanced cell motility and evasion from natural killer cell-mediated immune surveillance, as well as a marked advantage to form lung metastatic lesions in mice. Our findings reveal that CM increases the metastatic potential of breast cancer cells.
Keywords: IAP; anoikis; cancer biology; caspases; cell biology; confinement; human; mechanobiology; metastasis; mouse.
© 2022, Fanfone et al.
Conflict of interest statement
DF, ZW, JM, KB, DN, KW, AH, FV, FB, CJ, HH, ST, AH, GI No competing interests declared
Figures


Similar articles
-
Downregulation of COX-2 and CYP 4A signaling by isoliquiritigenin inhibits human breast cancer metastasis through preventing anoikis resistance, migration and invasion.Toxicol Appl Pharmacol. 2014 Oct 1;280(1):10-20. doi: 10.1016/j.taap.2014.07.018. Epub 2014 Aug 2. Toxicol Appl Pharmacol. 2014. PMID: 25094029
-
A TDO2-AhR signaling axis facilitates anoikis resistance and metastasis in triple-negative breast cancer.Cancer Res. 2015 Nov 1;75(21):4651-64. doi: 10.1158/0008-5472.CAN-15-2011. Epub 2015 Sep 11. Cancer Res. 2015. PMID: 26363006 Free PMC article.
-
Talin1 promotes tumor invasion and metastasis via focal adhesion signaling and anoikis resistance.Cancer Res. 2010 Mar 1;70(5):1885-95. doi: 10.1158/0008-5472.CAN-09-2833. Epub 2010 Feb 16. Cancer Res. 2010. PMID: 20160039 Free PMC article.
-
The Warburg effect in tumor progression: mitochondrial oxidative metabolism as an anti-metastasis mechanism.Cancer Lett. 2015 Jan 28;356(2 Pt A):156-64. doi: 10.1016/j.canlet.2014.04.001. Epub 2014 Apr 13. Cancer Lett. 2015. PMID: 24732809 Free PMC article. Review.
-
Targeting anoikis resistance in prostate cancer metastasis.Mol Aspects Med. 2010 Apr;31(2):205-14. doi: 10.1016/j.mam.2010.02.001. Epub 2010 Feb 11. Mol Aspects Med. 2010. PMID: 20153362 Free PMC article. Review.
Cited by
-
Lysyl hydroxylase LH1 promotes confined migration and metastasis of cancer cells by stabilizing Septin2 to enhance actin network.Mol Cancer. 2023 Jan 31;22(1):21. doi: 10.1186/s12943-023-01727-9. Mol Cancer. 2023. PMID: 36721170 Free PMC article.
-
CAR T Cell Locomotion in Solid Tumor Microenvironment.Cells. 2022 Jun 20;11(12):1974. doi: 10.3390/cells11121974. Cells. 2022. PMID: 35741103 Free PMC article. Review.
-
Keeping Cell Death Alive: An Introduction into the French Cell Death Research Network.Biomolecules. 2022 Jun 28;12(7):901. doi: 10.3390/biom12070901. Biomolecules. 2022. PMID: 35883457 Free PMC article. Review.
-
Therapeutic targeting of anoikis resistance in cutaneous melanoma metastasis.Front Cell Dev Biol. 2023 Apr 26;11:1183328. doi: 10.3389/fcell.2023.1183328. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37181747 Free PMC article. Review.
-
A novel risk model based on anoikis: Predicting prognosis and immune infiltration in cutaneous melanoma.Front Pharmacol. 2023 Jan 16;13:1090857. doi: 10.3389/fphar.2022.1090857. eCollection 2022. Front Pharmacol. 2023. PMID: 36726781 Free PMC article.
References
-
- Barrow AD, Edeling MA, Trifonov V, Luo J, Goyal P, Bohl B, Bando JK, Kim AH, Walker J, Andahazy M, Bugatti M, Melocchi L, Vermi W, Fremont DH, Cox S, Cella M, Schmedt C, Colonna M. Natural killer cells control tumor growth by sensing a growth factor. Cell. 2018;172:534–548. doi: 10.1016/j.cell.2017.11.037. - DOI - PMC - PubMed
-
- Berezovskaya O, Schimmer AD, Glinskii AB, Pinilla C, Hoffman RM, Reed JC, Glinsky GV. Increased expression of apoptosis inhibitor protein XIAP contributes to anoikis resistance of circulating human prostate cancer metastasis precursor cells. Cancer Research. 2005;65:2378–2386. doi: 10.1158/0008-5472.CAN-04-2649. - DOI - PubMed
Publication types
MeSH terms
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

