Flotillin 2 Facilitates the Infection of a Plant Virus in the Gut of Insect Vector
- PMID: 35254088
- PMCID: PMC9006895
- DOI: 10.1128/jvi.02140-21
Flotillin 2 Facilitates the Infection of a Plant Virus in the Gut of Insect Vector
Abstract
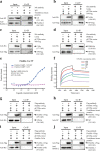
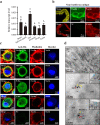
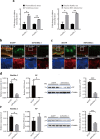
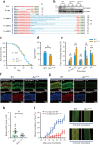
Most plant viruses require insect vectors for transmission. One of the key steps for the transmission of persistent-circulative plant viruses is overcoming the gut barrier to enter epithelial cells. To date, little has been known about viral cofactors in gut epithelial cells of insect vectors. Here, we identified flotillin 2 as a plasma membrane protein that facilitates the infection of rice stripe virus (RSV) in its vector, the small brown planthopper. Flotillin 2 displayed a prominent plasma membrane location in midgut epithelial cells. The nucleocapsid protein of RSV and flotillin 2 colocalized on gut microvilli, and a nanomolar affinity existed between the two proteins. Knockout of flotillin 2 impeded the entry of virions into epithelial cells, resulting in a 57% reduction of RSV levels in planthoppers. The knockout of flotillin 2 decreased disease incidence in rice plants fed by viruliferous planthoppers from 40% to 11.7%. Furthermore, flotillin 2 mediated the infection of southern rice black-streaked dwarf virus in its vector, the white-backed planthopper. This work implies the potential of flotillin 2 as a target for controlling the transmission of rice stripe disease. IMPORTANCE Plant viral diseases are a major threat to world agriculture. The transmission of 80% of plant viruses requires vector insects, and 54% of vector-borne plant viruses are persistent-circulative viruses, which must overcome the barriers of gut cells with the help of proteins on the cell surface. Here, we identified flotillin 2 as a membrane protein that mediates the cell entry of rice stripe virus in its vector insect, small brown planthopper. Flotillin 2 displays a prominent cellular membrane location in midgut cells and can specifically bind to virions. The loss of flotillin 2 impedes the entry of virions into the midgut cells of vector insects and substantially suppresses viral transmission to rice. Therefore, flotillin 2 may be a promising target gene for manipulation in vector insects to control the transmission of rice stripe disease and perhaps that of other rice virus diseases in the future.
Keywords: flotillin 2; insect vector; midgut; planthopper; plasma membrane protein; rice stripe virus; rice viral disease.
Conflict of interest statement
The authors declare no conflict of interest.
We have no conflicts of interest to declare.
Figures

Similar articles
-
Rice stripe virus-derived siRNAs play different regulatory roles in rice and in the insect vector Laodelphax striatellus.BMC Plant Biol. 2018 Oct 4;18(1):219. doi: 10.1186/s12870-018-1438-7. BMC Plant Biol. 2018. PMID: 30286719 Free PMC article.
-
Alternative Splicing Landscape of Small Brown Planthopper and Different Response of JNK2 Isoforms to Rice Stripe Virus Infection.J Virol. 2022 Jan 26;96(2):e0171521. doi: 10.1128/JVI.01715-21. Epub 2021 Nov 10. J Virol. 2022. PMID: 34757837 Free PMC article.
-
Characterization of protein-protein interactions between rice viruses and vector insects.Insect Sci. 2021 Aug;28(4):976-986. doi: 10.1111/1744-7917.12840. Epub 2020 Aug 3. Insect Sci. 2021. PMID: 32537916
-
Rice Responses and Resistance to Planthopper-Borne Viruses at Transcriptomic and Proteomic Levels.Curr Issues Mol Biol. 2016;19:43-52. Epub 2015 Sep 11. Curr Issues Mol Biol. 2016. PMID: 26363817 Review.
-
The small brown planthopper (Laodelphax striatellus) as a vector of the rice stripe virus.Arch Insect Biochem Physiol. 2023 Feb;112(2):e21992. doi: 10.1002/arch.21992. Epub 2022 Dec 27. Arch Insect Biochem Physiol. 2023. PMID: 36575628 Review.
Cited by
-
Plasmodesmata-associated Flotillin positively regulates broad-spectrum virus cell-to-cell trafficking.Plant Biotechnol J. 2024 May;22(5):1387-1401. doi: 10.1111/pbi.14274. Epub 2023 Dec 21. Plant Biotechnol J. 2024. PMID: 38130080 Free PMC article.
-
Key role of exportin 6 in exosome-mediated viral transmission from insect vectors to plants.Proc Natl Acad Sci U S A. 2022 Sep 6;119(36):e2207848119. doi: 10.1073/pnas.2207848119. Epub 2022 Aug 29. Proc Natl Acad Sci U S A. 2022. PMID: 36037368 Free PMC article.
References
-
- Toriyama S. 1986. Rice stripe virus: prototype of a new group of viruses that replicate in plants and insects. Microbiol Sci 3:347–351. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

