Evaluation of the clinical evolution and transmission of SARS-CoV-2 infection in cats by simulating natural routes of infection
- PMID: 35243589
- PMCID: PMC8893356
- DOI: 10.1007/s11259-022-09908-5
Evaluation of the clinical evolution and transmission of SARS-CoV-2 infection in cats by simulating natural routes of infection
Erratum in
-
Correction to: Evaluation of the clinical evolution and transmission of SARS-CoV-2 infection in cats by simulating natural routes of infection.Vet Res Commun. 2023 Jan;47(1):319. doi: 10.1007/s11259-022-09955-y. Vet Res Commun. 2023. PMID: 35699819 Free PMC article. No abstract available.
Abstract
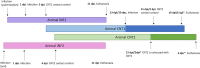
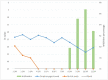
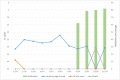
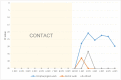
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative agent of the current pandemic disease denominated as Coronavirus Disease 2019 (COVID-19). Several studies suggest that the original source of this virus was a spillover from an animal reservoir and its subsequent adaptation to humans. Of all the different animals affected, cats are one of the most susceptible species. Moreover, several cases of natural infection in domestic and stray cats have been reported in the last few months. Although experimental infection assays have demonstrated that cats are successfully infected and can transmit the virus to other cats by aerosol, the conditions used for these experiments have not been specified in terms of ventilation. We have, therefore, evaluated the susceptibility of cats using routes of infection similar to those expected under natural conditions (exposure to a sneeze, cough, or contaminated environment) by aerosol and oral infection. We have also evaluated the transmission capacity among infected and naïve cats using different air exchange levels. Despite being infected using natural routes and shed virus for a long period, the cats did not transmit the virus to contact cats when air renovation features were employed. The infected animals also developed gross and histological lesions in several organs. These outcomes confirm that cats are at risk of infection when exposed to infected people, but do not transmit the virus to other cats with high rates of air renovation.
Keywords: Air renovation; Cats; Routes of infection; SARS-CoV-2; Transmission.
© 2022. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures



Similar articles
-
Experimental infection of domestic dogs and cats with SARS-CoV-2: Pathogenesis, transmission, and response to reexposure in cats.Proc Natl Acad Sci U S A. 2020 Oct 20;117(42):26382-26388. doi: 10.1073/pnas.2013102117. Epub 2020 Sep 29. Proc Natl Acad Sci U S A. 2020. PMID: 32994343 Free PMC article.
-
SARS-CoV-2 infection, disease and transmission in domestic cats.Emerg Microbes Infect. 2020 Dec;9(1):2322-2332. doi: 10.1080/22221751.2020.1833687. Emerg Microbes Infect. 2020. PMID: 33028154 Free PMC article.
-
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and humoral responses against different variants of concern in domestic pet animals and stray cats from North-Eastern Spain.Transbound Emerg Dis. 2022 Nov;69(6):3518-3529. doi: 10.1111/tbed.14714. Epub 2022 Oct 7. Transbound Emerg Dis. 2022. PMID: 36167932 Free PMC article.
-
Infection Dynamics, Pathogenesis, and Immunity to SARS-CoV-2 in Naturally Susceptible Animal Species.J Immunol. 2023 Oct 15;211(8):1195-1201. doi: 10.4049/jimmunol.2300378. J Immunol. 2023. PMID: 37782853 Free PMC article. Review.
-
[SARS-CoV-2 infections in cats, dogs, and other animal species: Findings on infection and data from Switzerland].Schweiz Arch Tierheilkd. 2021 Dec;163(12):821-835. doi: 10.17236/sat00329. Schweiz Arch Tierheilkd. 2021. PMID: 34881715 Review. German.
Cited by
-
Future trajectory of SARS-CoV-2: Constant spillover back and forth between humans and animals.Virus Res. 2023 Apr 15;328:199075. doi: 10.1016/j.virusres.2023.199075. Epub 2023 Feb 28. Virus Res. 2023. PMID: 36805410 Free PMC article. Review.
-
Comparative SARS-CoV-2 Omicron BA.5 variant and D614G-Wuhan strain infections in ferrets: insights into attenuation and disease progression during subclinical to mild COVID-19.Front Vet Sci. 2024 Aug 15;11:1435464. doi: 10.3389/fvets.2024.1435464. eCollection 2024. Front Vet Sci. 2024. PMID: 39211479 Free PMC article.
-
A useful tool for the safe diagnosis and control of the two main pandemics of the XXI century: COVID-19 and African Swine Fever disease.PLoS One. 2023 Mar 6;18(3):e0282632. doi: 10.1371/journal.pone.0282632. eCollection 2023. PLoS One. 2023. PMID: 36877705 Free PMC article.
-
Natural SARS-CoV- 2 infection in domestic cats and dogs of humans diagnosed with COVID-19 in Valle de Aburrá, Antioquia.Biomedica. 2022 Oct 31;42(Sp. 2):48-58. doi: 10.7705/biomedica.6407. Biomedica. 2022. PMID: 36322547 Free PMC article. English, Spanish.
-
SARS-CoV-2 Seroprevalence Studies in Pets, Spain.Emerg Infect Dis. 2023 Jun;29(6):1136-1142. doi: 10.3201/eid2906.221737. Epub 2023 Apr 17. Emerg Infect Dis. 2023. PMID: 37069624 Free PMC article.
References
-
- Bao L, Song Z, Xue J, Gao H, Liu J, Wang J, Guo Q, Zhao B, Qu Y, Qi F, Gong S, Liu M, Qi L, Li D, Han Y, Zhao W, Deng S, Liu Y, Xiang Z, Yang B, Deng W, Yu H, Cong Z, Wei Q, Xu J, Gao GF, Qin C. Susceptibility and Attenuated Transmissibility of SARS-CoV-2 in Domestic Cats. J Infect Dis. 2021 doi: 10.1093/infdis/jiab104. - DOI - PMC - PubMed
-
- Barroso-Arévalo S, Barneto A, Ramos ÁM, Rivera B, Sánchez R, Sánchez-Morales L, Pérez-Sancho M, Buendía A, Ferreras E, Ortiz-Menéndez JC, Moreno I, Serres C, Vela C, Risalde MÁ, Domínguez L, Sánchez-Vizcaíno JM. Large-scale study on virological and serological prevalence of SARS-CoV-2 in cats and dogs in Spain. Transbound Emerg Dis. 2021 doi: 10.1111/tbed.14366. - DOI - PMC - PubMed
-
- Bosco-Lauth AM, Hartwig AE, Porter SM, Gordy PW, Nehring M, Byas AD, VandeWoude S, Ragan IK, Maison RM, Bowen RA. Experimental infection of domestic dogs and cats with SARS-CoV-2: Pathogenesis, transmission, and response to reexposure in cats. Proc Natl Acad Sci USA. 2020;117(42):26382. doi: 10.1073/pnas.2013102117. - DOI - PMC - PubMed
-
- Chan JF, Zhang AJ, Yuan S, Poon VK, Chan CC, Lee AC, Chan WM, Fan Z, Tsoi HW, Wen L, Liang R, Cao J, Chen Y, Tang K, Luo C, Cai JP, Kok KH, Chu H, Chan KH, Sridhar S, Chen Z, Chen H, To KK, Yuen KY. Simulation of the Clinical and Pathological Manifestations of Coronavirus Disease 2019 (COVID-19) in a Golden Syrian Hamster Model: Implications for Disease Pathogenesis and Transmissibility. Clin Infect Dis. 2020;71(9):2428–2446. doi: 10.1093/cid/ciaa325. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

