The Promise of DNA Methylation in Understanding Multigenerational Factors in Autism Spectrum Disorders
- PMID: 35242170
- PMCID: PMC8886225
- DOI: 10.3389/fgene.2022.831221
The Promise of DNA Methylation in Understanding Multigenerational Factors in Autism Spectrum Disorders
Abstract
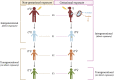
Autism spectrum disorder (ASD) is a group of neurodevelopmental disorders characterized by impairments in social reciprocity and communication, restrictive interests, and repetitive behaviors. Most cases of ASD arise from a confluence of genetic susceptibility and environmental risk factors, whose interactions can be studied through epigenetic mechanisms such as DNA methylation. While various parental factors are known to increase risk for ASD, several studies have indicated that grandparental and great-grandparental factors may also contribute. In animal studies, gestational exposure to certain environmental factors, such as insecticides, medications, and social stress, increases risk for altered behavioral phenotypes in multiple subsequent generations. Changes in DNA methylation, gene expression, and chromatin accessibility often accompany these altered behavioral phenotypes, with changes often appearing in genes that are important for neurodevelopment or have been previously implicated in ASD. One hypothesized mechanism for these phenotypic and methylation changes includes the transmission of DNA methylation marks at individual chromosomal loci from parent to offspring and beyond, called multigenerational epigenetic inheritance. Alternatively, intermediate metabolic phenotypes in the parental generation may confer risk from the original grandparental exposure to risk for ASD in grandchildren, mediated by DNA methylation. While hypothesized mechanisms require further research, the potential for multigenerational epigenetics assessments of ASD risk has implications for precision medicine as the field attempts to address the variable etiology and clinical signs of ASD by incorporating genetic, environmental, and lifestyle factors. In this review, we discuss the promise of multigenerational DNA methylation investigations in understanding the complex etiology of ASD.
Keywords: DNA methylation; autism spectrum disorder; epigenetics; metabolism; multigenerational; neurodevelopment; precision medicine; transgenerational.
Copyright © 2022 Mouat and LaSalle.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
[Epigenetics' implication in autism spectrum disorders: A review].Encephale. 2017 Aug;43(4):374-381. doi: 10.1016/j.encep.2016.07.007. Epub 2016 Sep 28. Encephale. 2017. PMID: 27692350 Review. French.
-
Association of Grandparental and Parental Age at Childbirth With Autism Spectrum Disorder in Children.JAMA Netw Open. 2020 Apr 1;3(4):e202868. doi: 10.1001/jamanetworkopen.2020.2868. JAMA Netw Open. 2020. PMID: 32293683 Free PMC article.
-
DNA methylation: a mechanism linking environmental chemical exposures to risk of autism spectrum disorders?Environ Epigenet. 2016 Mar;2(1):dvv012. doi: 10.1093/eep/dvv012. Epub 2016 Jan 30. Environ Epigenet. 2016. PMID: 27158529 Free PMC article.
-
Genetic and Epigenetic Etiology Underlying Autism Spectrum Disorder.J Clin Med. 2020 Mar 31;9(4):966. doi: 10.3390/jcm9040966. J Clin Med. 2020. PMID: 32244359 Free PMC article. Review.
-
DNA Methylation Associated with Mitochondrial Dysfunction in a South African Autism Spectrum Disorder Cohort.Autism Res. 2020 Jul;13(7):1079-1093. doi: 10.1002/aur.2310. Epub 2020 Jun 3. Autism Res. 2020. PMID: 32490597 Free PMC article.
Cited by
-
A scoping review of multigenerational impacts of grandparental exposures on mental health in grandchildren.Curr Environ Health Rep. 2023 Dec;10(4):369-382. doi: 10.1007/s40572-023-00413-8. Epub 2023 Nov 27. Curr Environ Health Rep. 2023. PMID: 38008881
-
Enhanced Expression of Human Endogenous Retroviruses, TRIM28 and SETDB1 in Autism Spectrum Disorder.Int J Mol Sci. 2022 May 25;23(11):5964. doi: 10.3390/ijms23115964. Int J Mol Sci. 2022. PMID: 35682642 Free PMC article.
-
Conceptualizing Epigenetics and the Environmental Landscape of Autism Spectrum Disorders.Genes (Basel). 2023 Aug 30;14(9):1734. doi: 10.3390/genes14091734. Genes (Basel). 2023. PMID: 37761876 Free PMC article. Review.
-
Generational synaptic functions of GABAA receptor β3 subunit deteriorations in an animal model of social deficit.J Biomed Sci. 2022 Jul 11;29(1):51. doi: 10.1186/s12929-022-00835-w. J Biomed Sci. 2022. PMID: 35821032 Free PMC article.
References
-
- Blanc M., Cormier B., Hyötyläinen T., Krauss M., Scherbak N., Cousin X., et al. (2020). Multi- and Transgenerational Effects Following Early-Life Exposure of Zebrafish to Permethrin and Coumarin 47: Impact on Growth, Fertility, Behavior and Lipid Metabolism. Ecotoxicology Environ. Saf. 205 (December), 111348. 10.1016/j.ecoenv.2020.111348 - DOI - PubMed

