Role of Dexamethasone and Methylprednisolone Corticosteroids in Coronavirus Disease 2019 Hospitalized Patients: A Review
- PMID: 35242118
- PMCID: PMC8886296
- DOI: 10.3389/fmicb.2022.813358
Role of Dexamethasone and Methylprednisolone Corticosteroids in Coronavirus Disease 2019 Hospitalized Patients: A Review
Abstract
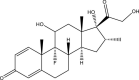
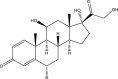
The WHO announced coronavirus disease 2019 (COVID-19) as a pandemic disease globally on March 11, 2020, after it emerged in China. The emergence of COVID-19 has lasted over a year, and despite promising vaccine reports that have been produced, we still have a long way to go until such remedies are accessible to everyone. The immunomodulatory strategy has been kept at the top priority for the research agenda for COVID-19. Corticosteroids have been used to modulate the immune response in a wide range of diseases for the last 70 years. These drugs have been shown to avoid and reduce inflammation in tissues and the bloodstream through non-genomic and genomic effects. Now, the use of corticosteroids increased the chance of survival and relief by combating the viral strong inflammatory impacts and has moved to the forefront in the management of patients seeking supplemental oxygen. The goal of this review is to illuminate dexamethasone and methylprednisolone, i.e., in terms of their chemical and physical properties, role in COVID-19 patients suffering from pneumonia, the proposed mode of action in COVID-19, pharmacokinetics, pharmacodynamics, clinical outcomes in immunocompromised populations with COVID-19, interaction with other drugs, and contradiction to explore the trends and perspectives for future research. Literature was searched from scientific databases such as Science Direct, Wiley, Springer, PubMed, and books for the preparation of this review. The RECOVERY trial, a massive, multidisciplinary, randomized, and open-label trial, is mainly accountable for recommendations over the usage of corticosteroids in COVID-19 patients. The corticosteroids such as dexamethasone and methylprednisolone in the form of medication have anti-inflammatory, analgesic, and anti-allergic characteristics, including the ability to inhibit the immune system. These drugs are also recommended for treating symptoms of multiple ailments such as rheumatic and autoimmune diseases, leukemia, multiple myeloma, and Hodgkin's and non-Hodgkin's lymphoma along with other drugs. Toxicology studies proved them safe usually at low dosage via oral or other routes.
Keywords: COVID-19; corticosteroids; dexamethasone; inflammatory impacts; methylprednisolone; pharmacodynamics; pharmacokinetics.
Copyright © 2022 Mehta, Rolta, Mehta, Kaushik, Choi and Kaushik.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Corticosteroids for COVID-19.J Intensive Med. 2021 Feb 5;1(1):14-25. doi: 10.1016/j.jointm.2021.01.002. eCollection 2021 Jul. J Intensive Med. 2021. PMID: 36943816 Free PMC article. Review.
-
Does methylprednisolone reduce the mortality risk in hospitalized COVID-19 patients? A meta-analysis of randomized control trials.Expert Rev Respir Med. 2021 Aug;15(8):1049-1055. doi: 10.1080/17476348.2021.1925546. Epub 2021 Jul 2. Expert Rev Respir Med. 2021. PMID: 33945381
-
Corticosteroids for treatment of COVID-19: effect, evidence, expectation and extent.Beni Suef Univ J Basic Appl Sci. 2021;10(1):78. doi: 10.1186/s43088-021-00165-0. Epub 2021 Nov 4. Beni Suef Univ J Basic Appl Sci. 2021. PMID: 34751250 Free PMC article. Review.
-
Corticosteroids for treating optic neuritis.Cochrane Database Syst Rev. 2015 Aug 14;2015(8):CD001430. doi: 10.1002/14651858.CD001430.pub4. Cochrane Database Syst Rev. 2015. PMID: 26273799 Free PMC article. Review.
-
Efficacy and safety profile of corticosteroids and non-steroidal anti-inflammatory drugs in COVID-19 management: A narrative review.Front Pharmacol. 2022 Dec 1;13:1063246. doi: 10.3389/fphar.2022.1063246. eCollection 2022. Front Pharmacol. 2022. PMID: 36532785 Free PMC article. Review.
Cited by
-
A Comprehensive Review on the Therapeutic Potential of Curcuma longa Linn. in Relation to its Major Active Constituent Curcumin.Front Pharmacol. 2022 Mar 25;13:820806. doi: 10.3389/fphar.2022.820806. eCollection 2022. Front Pharmacol. 2022. PMID: 35401176 Free PMC article. Review.
-
Clinical Characteristics and Outcome of Hospitalized COVID-19 Patients Treated with Standard Dose of Dexamethasone or High Dose of Methylprednisolone.Biomedicines. 2022 Jun 29;10(7):1548. doi: 10.3390/biomedicines10071548. Biomedicines. 2022. PMID: 35884852 Free PMC article.
-
A comparative study between methylprednisolone versus dexamethasone as an initial anti-inflammatory treatment of moderate COVID-19 pneumonia: an open-label randomized controlled trial.BMC Pulm Med. 2024 Nov 11;24(1):562. doi: 10.1186/s12890-024-03364-4. BMC Pulm Med. 2024. PMID: 39529039 Free PMC article. Clinical Trial.
-
Short- and long-term T cell and antibody responses following dexamethasone treatment in COVID-19.JCI Insight. 2023 Apr 24;8(8):e166711. doi: 10.1172/jci.insight.166711. JCI Insight. 2023. PMID: 36881474 Free PMC article.
-
Clinical effect of early administration of tocilizumab following the initiation of corticosteroid therapy for patients with COVID-19.J Infect Chemother. 2022 Dec;28(12):1639-1644. doi: 10.1016/j.jiac.2022.08.021. Epub 2022 Aug 31. J Infect Chemother. 2022. PMID: 36057415 Free PMC article.
References
-
- Annane D., Pastores S. M., Arlt W., Balk R. A., Beishuizen A., Briegel J., et al. (2017). Critical illness-related corticosteroid insufficiency (CIRCI): a narrative review from a Multispecialty Task Force of the Society of Critical Care Medicine (SCCM) and the European Society of Intensive Care Medicine (ESICM). Intensive Care Med. 43 1781–1792. 10.1007/s00134-017-4914-x - DOI - PubMed
-
- Bieber T., Vick K., Fölster-Holst R., Belloni-Fortina A., Städtler G., Worm M., et al. (2007). Efficacy and safety of methylprednisolone aceponate ointment 0.1% compared to tacrolimus 0.03% in children and adolescents with an acute flare of severe atopic dermatitis. Allergy 62 184–189. 10.1111/j.1398-9995.2006.01269.x - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

