Glutathione deficiency decreases lipid droplet stores and increases reactive oxygen species in mouse oocytes†
- PMID: 35238901
- PMCID: PMC9198951
- DOI: 10.1093/biolre/ioac032
Glutathione deficiency decreases lipid droplet stores and increases reactive oxygen species in mouse oocytes†
Abstract
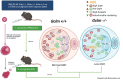
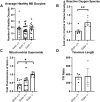
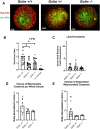
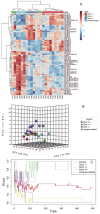
Glutathione (GSH) is a tripeptide thiol antioxidant that has been shown to be important to overall reproductive health. Glutamate cysteine ligase, the rate-limiting enzyme in GSH synthesis consists of a catalytic and a modifier (GCLM) subunit. We previously showed that oxidative stress in the ovary and oocytes of Gclm-/- mice is associated with accelerated age-related decline in ovarian follicles and decreased female fertility due to preimplantation embryonic mortality. Mammalian preimplantation development is a highly regulated and energy-intensive process that primarily relies on coordination between lipid droplets (LDs) and mitochondria to maintain cellular homeostasis. In this study, we hypothesized that GSH deficiency in oocytes increases oxidative stress, leading to increased mitochondrial dysfunction and decreased LD consumption, thereby decreasing oocyte developmental competence. We observed that Gclm-/- oocytes have increased oxidative stress, primarily in the form of mitochondrial superoxide and decreased subcortical mitochondrial clusters. Further, Gclm-/- oocytes have decreased mitochondrial membrane potential (ΔΨm) compared with Gclm+/+. We surmise this is likely due to the decreased availability of LDs, as we observed a significant decrease in LD content in Gclm-/- oocytes compared with Gclm+/+. The decreased oocyte LD content is likely related to an altered serum lipidome, with Gclm-/- serum having relatively lower unsaturated fatty acids and triglycerides than that of Gclm+/+ and Gclm+/- females. Altogether these data support that decreased LDs and increased oxidative stress are primary drivers of decreased oocyte developmental competence in GSH-deficient oocytes.
Keywords: glutathione; lipid droplets; lipidomics; mitochondria; oocyte; oxidative stress.
© The Author(s) 2022. Published by Oxford University Press on behalf of Society for the Study of Reproduction. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

Similar articles
-
Antioxidant supplementation partially rescues accelerated ovarian follicle loss, but not oocyte quality, of glutathione-deficient mice†.Biol Reprod. 2020 Apr 24;102(5):1065-1079. doi: 10.1093/biolre/ioaa009. Biol Reprod. 2020. PMID: 31950131 Free PMC article.
-
Lack of maternal glutamate cysteine ligase modifier subunit (Gclm) decreases oocyte glutathione concentrations and disrupts preimplantation development in mice.Endocrinology. 2011 Jul;152(7):2806-15. doi: 10.1210/en.2011-0207. Epub 2011 May 10. Endocrinology. 2011. PMID: 21558310 Free PMC article.
-
Decreased glutathione synthesis in granulosa cells, but not oocytes, of growing follicles decreases fertility in mice†.Biol Reprod. 2024 Nov 11;111(5):1097-1106. doi: 10.1093/biolre/ioae124. Biol Reprod. 2024. PMID: 39151022
-
Homeostatic regulation of lipid droplet content in mammalian oocytes and embryos.Reproduction. 2021 Oct 28;162(6):R99-R109. doi: 10.1530/REP-21-0238. Reproduction. 2021. PMID: 34715675 Review.
-
Modulating GSH synthesis using glutamate cysteine ligase transgenic and gene-targeted mice.Drug Metab Rev. 2008;40(3):465-77. doi: 10.1080/03602530802186587. Drug Metab Rev. 2008. PMID: 18642143 Review.
Cited by
-
Gestational Benzo[a]pyrene Exposure Destroys F1 Ovarian Germ Cells Through Mitochondrial Apoptosis Pathway and Diminishes Surviving Oocyte Quality.Toxicol Sci. 2022 Oct 27;190(1):23-40. doi: 10.1093/toxsci/kfac086. Toxicol Sci. 2022. PMID: 35993611 Free PMC article.
-
Differentially Expressed Genes in Response to a Squalene-Supplemented Diet Are Accurate Discriminants of Porcine Non-Alcoholic Steatohepatitis.Int J Mol Sci. 2023 Aug 8;24(16):12552. doi: 10.3390/ijms241612552. Int J Mol Sci. 2023. PMID: 37628732 Free PMC article.
-
An Overview of Reactive Oxygen Species Damage Occurring during In Vitro Bovine Oocyte and Embryo Development and the Efficacy of Antioxidant Use to Limit These Adverse Effects.Animals (Basel). 2024 Jan 21;14(2):330. doi: 10.3390/ani14020330. Animals (Basel). 2024. PMID: 38275789 Free PMC article. Review.
-
Role of nuclear factor erythroid 2-related factor 2 (Nrf2) in female and male fertility.Heliyon. 2024 Apr 16;10(9):e29752. doi: 10.1016/j.heliyon.2024.e29752. eCollection 2024 May 15. Heliyon. 2024. PMID: 38720768 Free PMC article. Review.
References
-
- Dalton TP, Dieter MZ, Y Y, Shertzer HG, Nebert DW. Knockout of the mouse glutamate cysteine ligase catalytic subunit (Gclc) gene: embryonic lethal when homozygous, and proposed model for moderate glutathione deficiency when heterozygous. Biochem Biophys Res Commun 2000; 279:324–329. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

