Mechanisms Underlying Influence of Bioelectricity in Development
- PMID: 35237593
- PMCID: PMC8883286
- DOI: 10.3389/fcell.2022.772230
Mechanisms Underlying Influence of Bioelectricity in Development
Abstract
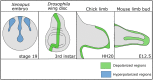
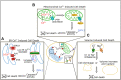
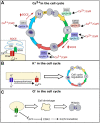
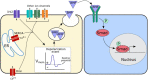
To execute the intricate process of development, cells coordinate across tissues and organs to determine where each cell divides and differentiates. This coordination requires complex communication between cells. Growing evidence suggests that bioelectrical signals controlled via ion channels contribute to cell communication during development. Ion channels collectively regulate the transmembrane potential of cells, and their function plays a conserved role in the development of organisms from flies to humans. Spontaneous calcium oscillations can be found in nearly every cell type and tissue, and disruption of these oscillations leads to defects in development. However, the mechanism by which bioelectricity regulates development is still unclear. Ion channels play essential roles in the processes of cell death, proliferation, migration, and in each of the major canonical developmental signaling pathways. Previous reviews focus on evidence for one potential mechanism by which bioelectricity affects morphogenesis, but there is evidence that supports multiple different mechanisms which are not mutually exclusive. Evidence supports bioelectricity contributing to development through multiple different mechanisms. Here, we review evidence for the importance of bioelectricity in morphogenesis and provide a comprehensive review of the evidence for several potential mechanisms by which ion channels may act in developmental processes.
Keywords: apoptosis; bioelectricity; ion channels; prolifieration; signaling; signaling pathways.
Copyright © 2022 George and Bates.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Ion channels in development and cancer.Annu Rev Cell Dev Biol. 2015;31:231-47. doi: 10.1146/annurev-cellbio-100814-125338. Annu Rev Cell Dev Biol. 2015. PMID: 26566112 Review.
-
Bioelectricity in dental medicine: a narrative review.Biomed Eng Online. 2024 Jan 3;23(1):3. doi: 10.1186/s12938-023-01189-6. Biomed Eng Online. 2024. PMID: 38172866 Free PMC article. Review.
-
On the coupling of mechanics with bioelectricity and its role in morphogenesis.J R Soc Interface. 2020 Jun;17(167):20200177. doi: 10.1098/rsif.2020.0177. Epub 2020 Jun 3. J R Soc Interface. 2020. PMID: 32486953 Free PMC article.
-
Nanotechnology and Cancer Bioelectricity: Bridging the Gap Between Biology and Translational Medicine.Adv Sci (Weinh). 2024 Jan;11(1):e2304110. doi: 10.1002/advs.202304110. Epub 2023 Nov 20. Adv Sci (Weinh). 2024. PMID: 37984883 Free PMC article. Review.
-
Ion Channel Contributions to Wing Development in Drosophila melanogaster.G3 (Bethesda). 2019 Apr 9;9(4):999-1008. doi: 10.1534/g3.119.400028. G3 (Bethesda). 2019. PMID: 30733380 Free PMC article.
Cited by
-
Quantum Biology and the Potential Role of Entanglement and Tunneling in Non-Targeted Effects of Ionizing Radiation: A Review and Proposed Model.Int J Mol Sci. 2023 Nov 17;24(22):16464. doi: 10.3390/ijms242216464. Int J Mol Sci. 2023. PMID: 38003655 Free PMC article. Review.
-
Bioelectricity in Developmental Patterning and Size Control: Evidence and Genetically Encoded Tools in the Zebrafish Model.Cells. 2023 Apr 13;12(8):1148. doi: 10.3390/cells12081148. Cells. 2023. PMID: 37190057 Free PMC article. Review.
-
Bioelectric Potential in Next-Generation Organoids: Electrical Stimulation to Enhance 3D Structures of the Central Nervous System.Front Cell Dev Biol. 2022 May 17;10:901652. doi: 10.3389/fcell.2022.901652. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35656553 Free PMC article. Review.
-
The scale of zebrafish pectoral fin buds is determined by intercellular K+ levels and consequent Ca2+-mediated signaling via retinoic acid regulation of Rcan2 and Kcnk5b.PLoS Biol. 2024 Mar 25;22(3):e3002565. doi: 10.1371/journal.pbio.3002565. eCollection 2024 Mar. PLoS Biol. 2024. PMID: 38527087 Free PMC article.
-
Modeling non-genetic information dynamics in cells using reservoir computing.iScience. 2024 Mar 28;27(4):109614. doi: 10.1016/j.isci.2024.109614. eCollection 2024 Apr 19. iScience. 2024. PMID: 38632985 Free PMC article.
References
-
- Adams D. S., Uzel S. G. M., Akagi J., Wlodkowic D., Andreeva V., Yelick P. C., et al. (2016). Bioelectric Signalling via Potassium Channels: a Mechanism for Craniofacial Dysmorphogenesis in KCNJ2-Associated Andersen-Tawil Syndrome. J. Physiol. 594 (12), 3245–3270. 10.1113/jp271930 - DOI - PMC - PubMed
-
- Amigorena S., Choquet D., Teillaud J. L., Korn H., Fridman W. H. (1990). Ion Channel Blockers Inhibit B Cell Activation at a Precise Stage of the G1 Phase of the Cell Cycle. Possible Involvement of K+ Channels. J. Immunol. 144 (6), 2038–2045. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

