Comprehensive analysis of SPAG1 expression as a prognostic and predictive biomarker in acute myeloid leukemia by integrative bioinformatics and clinical validation
- PMID: 35227274
- PMCID: PMC8886923
- DOI: 10.1186/s12920-022-01193-0
Comprehensive analysis of SPAG1 expression as a prognostic and predictive biomarker in acute myeloid leukemia by integrative bioinformatics and clinical validation
Abstract
Background: Recently, an increasing number of studies have reported that sperm-associated antigen (SPAG) proteins play crucial roles in solid tumorigenesis, and may serve as potentially helpful biomarkers for cancer diagnosis and prognosis. However, very few studies systematically investigated the expression of SPAG family members and their clinical significance in acute myeloid leukemia (AML).
Methods: The expression of SPAGs and their prognostic significance in AML were determined by a systematic analysis on data gathered from public databases, and the results were validated in clinical samples.
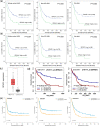
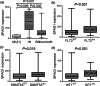
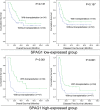
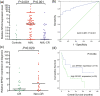
Results: Using public data, we identified only increased SPAG1 expression negatively associated with survival in AML by Cox regression (P < 0.001) and Kaplan-Meier analysis (P < 0.001). The prognostic value of SPAG1 expression was further confirmed in other independent cohorts. Clinically, higher SPAG1 expression was significantly correlated with white blood cell counts (P = 0.014) and French-American-British (FAB) subtypes (P = 0.024). Moreover, higher SPAG1 expression was more common in + 8 patients (P = 0.034), rarely found with t(8;21) (P = 0.014), and correlated with FLT3 (P < 0.001) and DNMT3A mutations (P = 0.001). Despite these associations, multivariate analysis confirmed the independent prognostic value of SPAG1 expression in AML (P < 0.001). Notably, AML patients with higher SPAG1 expression may benefit from hematopoietic stem cell transplantation (HSCT), whereas patients with lower SPAG1 expression appeared less likely to benefit. Finally, we further validated that SPAG1 expression was significantly increased in newly diagnosed AML patients compared with normal controls (P < 0.001) and with AML patients who achieved complete remission (P < 0.001). Additionally, SPAG1 expression could act as a potentially helpful biomarker for the diagnosis and prognosis of AML (P < 0.001 and = 0.034, respectively).
Conclusions: Our findings demonstrated that SPAG1 overexpression may serve as an independent prognostic biomarker and may guide the choice between HSCT and chemotherapy in patients with AML.
Keywords: AML; Bioinformatics; Expression; Prognosis; SPAG1.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
SPAG1 promotes the development of AML by activating the ERK/MAPK signaling pathway and affects the chemotherapy sensitivity of venetoclax.Neoplasma. 2022 Sep;69(5):1108-1118. doi: 10.4149/neo_2022_220415N416. Epub 2022 Aug 12. Neoplasma. 2022. PMID: 35951456
-
Expression and prognosis analysis of DNMT family in acute myeloid leukemia.Aging (Albany NY). 2020 Jun 26;12(14):14677-14690. doi: 10.18632/aging.103520. Epub 2020 Jun 26. Aging (Albany NY). 2020. PMID: 32597790 Free PMC article.
-
NEDD9 overexpression: Prognostic and guidance value in acute myeloid leukaemia.J Cell Mol Med. 2021 Oct;25(19):9331-9339. doi: 10.1111/jcmm.16870. Epub 2021 Aug 25. J Cell Mol Med. 2021. PMID: 34432355 Free PMC article.
-
CAPN1 is a novel biomaker of patients with AML based on comprehensive analysis.Biotechnol Genet Eng Rev. 2024 Dec;40(4):3884-3900. doi: 10.1080/02648725.2023.2204688. Epub 2023 Apr 28. Biotechnol Genet Eng Rev. 2024. PMID: 37114994
-
Disparities in acute myeloid leukemia treatments and outcomes.Curr Opin Hematol. 2024 Mar 1;31(2):58-63. doi: 10.1097/MOH.0000000000000797. Epub 2023 Dec 7. Curr Opin Hematol. 2024. PMID: 38059809 Review.
Cited by
-
Prognostic Significance of Dual-Specificity Phosphatase 23 Expression in Acute Myeloid Leukemia.J Blood Med. 2024 Feb 7;15:35-50. doi: 10.2147/JBM.S437400. eCollection 2024. J Blood Med. 2024. PMID: 38344181 Free PMC article.
-
Comprehensive analysis of ID genes reveals the clinical and prognostic value of ID3 expression in acute myeloid leukemia using bioinformatics identification and experimental validation.BMC Cancer. 2022 Nov 29;22(1):1229. doi: 10.1186/s12885-022-10352-6. BMC Cancer. 2022. PMID: 36443709 Free PMC article.
-
T cell-mediated tumor killing sensitivity gene signature-based prognostic score for acute myeloid leukemia.Discov Oncol. 2024 Apr 15;15(1):121. doi: 10.1007/s12672-024-00962-w. Discov Oncol. 2024. PMID: 38619693 Free PMC article.
-
Transcriptome Analysis of Beta-Catenin-Related Genes in CD34+ Haematopoietic Stem and Progenitor Cells from Patients with AML.Mediterr J Hematol Infect Dis. 2024 Jul 1;16(1):e2024058. doi: 10.4084/MJHID.2024.058. eCollection 2024. Mediterr J Hematol Infect Dis. 2024. PMID: 38984092 Free PMC article.
References
-
- Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, Dombret H, Ebert BL, Fenaux P, Larson RA, Levine RL, Lo-Coco F, Naoe T, Niederwieser D, Ossenkoppele GJ, Sanz M, Sierra J, Tallman MS, Tien HF, Wei AH, Löwenberg B, Bloomfield CD. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447. doi: 10.1182/blood-2016-08-733196. - DOI - PMC - PubMed
-
- Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M, Arber VJW, DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M, Vardiman JW, The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi: 10.1182/blood-2016-03-643544. - DOI - PubMed
-
- Burd A, Levine RL, Ruppert AS, Mims AS, Borate U, Stein EM, Patel P, Baer MR, Stock W, Deininger M, Blum W, Schiller G, Olin R, Litzow M, Foran J, Lin TL, Ball B, Boyiadzis M, Traer E, Odenike O, Arellano M, Walker A, Duong VH, Kovacsovics T, Collins R, Shoben AB, Heerema NA, Foster MC, Vergilio JA, Brennan T, Vietz C, Severson E, Miller M, Rosenberg L, Marcus S, Yocum A, Chen T, Stefanos M, Druker B, Byrd JC. Precision medicine treatment in acute myeloid leukemia using prospective genomic profiling: feasibility and preliminary efficacy of the Beat AML Master Trial. Nat Med. 2020;26(12):1852–1858. doi: 10.1038/s41591-020-1089-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

