A Viral Long Non-Coding RNA Protects against Cell Death during Human Cytomegalovirus Infection of CD14+ Monocytes
- PMID: 35215840
- PMCID: PMC8874509
- DOI: 10.3390/v14020246
A Viral Long Non-Coding RNA Protects against Cell Death during Human Cytomegalovirus Infection of CD14+ Monocytes
Abstract
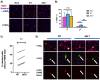
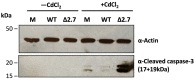
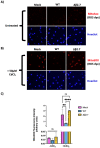
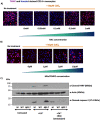
Long non-coding RNA β2.7 is the most highly transcribed viral gene during latent human cytomegalovirus (HCMV) infection. However, as yet, no function has ever been ascribed to β2.7 during HCMV latency. Here we show that β2.7 protects against apoptosis induced by high levels of reactive oxygen species (ROS) in infected monocytes, which routinely support latent HCMV infection. Monocytes infected with a wild-type (WT) virus, but not virus deleted for the β2.7 gene (Δβ2.7), are protected against mitochondrial stress and subsequent apoptosis. Protected monocytes display lower levels of ROS and additionally, stress-induced death in the absence of β2.7 can be reversed by an antioxidant which reduces ROS levels. Furthermore, we show that infection with WT but not Δβ2.7 virus results in strong upregulation of a cellular antioxidant enzyme, superoxide dismutase 2 (SOD2) in CD14+ monocytes. These observations identify a role for the β2.7 viral transcript, the most abundantly expressed viral RNA during latency but for which no latency-associated function has ever been ascribed, and demonstrate a novel way in which HCMV protects infected monocytes from pro-death signals to optimise latent carriage.
Keywords: apoptosis; human cytomegalovirus; latency; long non-coding RNA; oxidative stress; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The Human Cytomegalovirus β2.7 Long Non-Coding RNA Prevents Induction of Reactive Oxygen Species to Maintain Viral Gene Silencing during Latency.Int J Mol Sci. 2022 Sep 20;23(19):11017. doi: 10.3390/ijms231911017. Int J Mol Sci. 2022. PMID: 36232315 Free PMC article.
-
Human cytomegalovirus modulates monocyte-mediated innate immune responses during short-term experimental latency in vitro.J Virol. 2014 Aug;88(16):9391-405. doi: 10.1128/JVI.00934-14. Epub 2014 Jun 11. J Virol. 2014. PMID: 24920803 Free PMC article.
-
Latency-Associated Expression of Human Cytomegalovirus US28 Attenuates Cell Signaling Pathways To Maintain Latent Infection.mBio. 2017 Dec 5;8(6):e01754-17. doi: 10.1128/mBio.01754-17. mBio. 2017. PMID: 29208743 Free PMC article.
-
Human cytomegalovirus-encoded MicroRNAs: A master regulator of latent infection.Infect Genet Evol. 2020 Mar;78:104119. doi: 10.1016/j.meegid.2019.104119. Epub 2019 Nov 15. Infect Genet Evol. 2020. PMID: 31740397 Review.
-
[Latency and reactivation of HCMV].Nihon Rinsho. 2006 Mar;64 Suppl 3:435-9. Nihon Rinsho. 2006. PMID: 16615510 Review. Japanese. No abstract available.
Cited by
-
The Human Cytomegalovirus β2.7 Long Non-Coding RNA Prevents Induction of Reactive Oxygen Species to Maintain Viral Gene Silencing during Latency.Int J Mol Sci. 2022 Sep 20;23(19):11017. doi: 10.3390/ijms231911017. Int J Mol Sci. 2022. PMID: 36232315 Free PMC article.
-
Human Cytomegalovirus Induced Aberrant Expression of Non-coding RNAs.Front Microbiol. 2022 Jun 13;13:918213. doi: 10.3389/fmicb.2022.918213. eCollection 2022. Front Microbiol. 2022. PMID: 35770158 Free PMC article. Review.
-
Shaping the host cell environment with viral noncoding RNAs.Semin Cell Dev Biol. 2023 Sep 15;146:20-30. doi: 10.1016/j.semcdb.2022.12.008. Epub 2022 Dec 28. Semin Cell Dev Biol. 2023. PMID: 36581481 Free PMC article. Review.
-
Functional and molecular dissection of HCMV long non-coding RNAs.Sci Rep. 2022 Nov 11;12(1):19303. doi: 10.1038/s41598-022-23317-3. Sci Rep. 2022. PMID: 36369338 Free PMC article.
-
High-Risk Oncogenic Human Cytomegalovirus.Viruses. 2022 Nov 7;14(11):2462. doi: 10.3390/v14112462. Viruses. 2022. PMID: 36366560 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

