Ex Vivo Evaluation of Mucosal Responses to Vaccination with ALVAC and AIDSVAX of Non-Human Primates
- PMID: 35214645
- PMCID: PMC8879115
- DOI: 10.3390/vaccines10020187
Ex Vivo Evaluation of Mucosal Responses to Vaccination with ALVAC and AIDSVAX of Non-Human Primates
Abstract
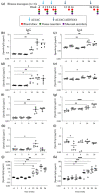
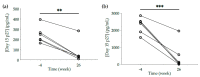
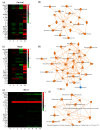
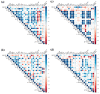
Non-human primates (NHPs) remain the most relevant challenge model for the evaluation of HIV vaccine candidates; however, discrepancies with clinical trial results have emphasized the need to further refine the NHP model. Furthermore, classical evaluation of vaccine candidates is based on endpoints measured systemically. We assessed the mucosal responses elicited upon vaccination with ALVAC and AIDSVAX using ex vivo Rhesus macaque mucosal tissue explant models. Following booster immunization with ALVAC/AIDSVAX, anti-gp120 HIV-1CM244-specific IgG and IgA were detected in culture supernatant cervicovaginal and colorectal tissue explants, as well as systemically. Despite protection from ex vivo viral challenge, no neutralization was observed with tissue explant culture supernatants. Priming with ALVAC induced distinct cytokine profiles in cervical and rectal tissue. However, ALVAC/AIDSVAX boosts resulted in similar modulations in both mucosal tissues with a statistically significant decrease in cytokines linked to inflammatory responses and lymphocyte differentiation. With ALVAC/AIDSVAX boosts, significant correlations were observed between cytokine levels and specific IgA in cervical explants and specific IgG and IgA in rectal tissue. The cytokine secretome revealed differences between vaccination with ALVAC and ALVAC/AIDSVAX not previously observed in mucosal tissues and distinct from the systemic response, which could represent a biosignature of the vaccine combination.
Keywords: HIV-1; mucosal immunology; vaccine.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Characterization of HIV-1 gp120 antibody specificities induced in anogenital secretions of RV144 vaccine recipients after late boost immunizations.PLoS One. 2018 Apr 27;13(4):e0196397. doi: 10.1371/journal.pone.0196397. eCollection 2018. PLoS One. 2018. PMID: 29702672 Free PMC article. Clinical Trial.
-
ALVAC-HIV and AIDSVAX B/E vaccination induce improved immune responses compared with AIDSVAX B/E vaccination alone.JCI Insight. 2023 May 8;8(9):e167664. doi: 10.1172/jci.insight.167664. JCI Insight. 2023. PMID: 37154156 Free PMC article.
-
Tissue memory B cell repertoire analysis after ALVAC/AIDSVAX B/E gp120 immunization of rhesus macaques.JCI Insight. 2016 Dec 8;1(20):e88522. doi: 10.1172/jci.insight.88522. JCI Insight. 2016. PMID: 27942585 Free PMC article.
-
ALVAC-HIV vaccines: clinical trial experience focusing on progress in vaccine development.Expert Rev Vaccines. 2004 Aug;3(4 Suppl):S99-104. doi: 10.1586/14760584.3.4.s99. Expert Rev Vaccines. 2004. PMID: 15285709 Review.
-
New concepts in HIV-1 vaccine development.Curr Opin Immunol. 2016 Aug;41:39-46. doi: 10.1016/j.coi.2016.05.011. Epub 2016 Jun 3. Curr Opin Immunol. 2016. PMID: 27268856 Free PMC article. Review.
Cited by
-
Pre-clinical evaluation of antiproteases as potential candidates for HIV-1 pre-exposure prophylaxis.Front Reprod Health. 2022 Nov 21;4:998913. doi: 10.3389/frph.2022.998913. eCollection 2022. Front Reprod Health. 2022. PMID: 36478892 Free PMC article.
References
-
- Buchbinder S.P., Mehrotra D.V., Duerr A., Fitzgerald D.W., Mogg R., Li D., Gilbert P.B., Lama J.R., Marmor M., Del Rio C., et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): A double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. - DOI - PMC - PubMed
-
- Chen X., Zhou T., Schmidt S., Duan H., Cheng C., Chuang G.-Y., Gu Y., Louder M., Lin B., Shen C.-H., et al. Vaccination induces maturation of diverse unmutated VRC01-class precursors to HIV-1 broadly neutralizing antibodies in an Ig-humanized mouse model. In Proceedings of the 4th HIV Research for Prevention conference (HIVR4P//Virtual), 27–28 January–3–4 February 2021. J. Int. AIDS Soc. 2021;24:e25659. doi: 10.1002/jia2.25659. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

