Delivery of Intravenously Administered Antibodies Targeting Alzheimer's Disease-Relevant Tau Species into the Brain Based on Receptor-Mediated Transcytosis
- PMID: 35214143
- PMCID: PMC8876001
- DOI: 10.3390/pharmaceutics14020411
Delivery of Intravenously Administered Antibodies Targeting Alzheimer's Disease-Relevant Tau Species into the Brain Based on Receptor-Mediated Transcytosis
Abstract
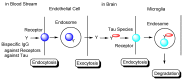
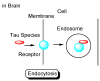
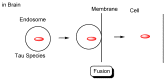
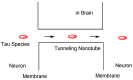
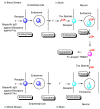
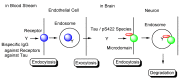
Alzheimer's disease (AD) is a neurodegenerative disease that causes memory loss, cognitive decline, and eventually dementia. The etiology of AD and its pathological mechanisms remain unclear due to its complex pathobiology. At the same time, the number of patients with AD is increasing worldwide. However, no therapeutic agents for AD are currently available for definitive care. Several phase 3 clinical trials using agents targeting amyloid β (Aβ) and its related molecules have failed, with the exception of aducanumab, an anti-Aβ monoclonal antibody (mAb), clinically approved by the US Food and Drug Administration in 2021, which could be modified for AD drug development due to controversial approval. Neurofibrillary tangles (NFTs) composed of tau rather than senile plaques composed of Aβ are correlated with AD pathogenesis. Moreover, Aβ and tau pathologies initially proceed independently. At a certain point in the progression of AD symptoms, the Aβ pathology is involved in the alteration and spreading of the tau pathology. Therefore, tau-targeting therapies have attracted the attention of pharmaceutical scientists, as well as Aβ-targeting therapies. In this review, I introduce the implementations and potential of AD immunotherapy using intravenously administered anti-tau and anti-receptor bispecific mAbs. These cross the blood-brain barrier (BBB) based on receptor-mediated transcytosis and are subsequently cleared by microglia based on Fc-mediated endocytosis after binding to tau and lysosomal degradation.
Keywords: Alzheimer’s disease; Alzheimer’s disease-relevant tau species; anti-tau and anti-receptor bispecific monoclonal antibodies; drug delivery into the brain; immunotherapy; interactions between Aβ and tau; tau clearance in microglia; tau clearance in neurons; temporal-spatial pathological Aβ and tau distribution; transendothelium based on receptor-mediated transcytosis.
Conflict of interest statement
The author declares no conflict of interest.
Figures


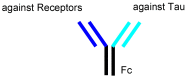
Similar articles
-
Impact of Anti-amyloid-β Monoclonal Antibodies on the Pathology and Clinical Profile of Alzheimer's Disease: A Focus on Aducanumab and Lecanemab.Front Aging Neurosci. 2022 Apr 12;14:870517. doi: 10.3389/fnagi.2022.870517. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35493943 Free PMC article. Review.
-
Immunotherapy for Alzheimer's disease: from anti-β-amyloid to tau-based immunization strategies.Immunotherapy. 2012 Feb;4(2):213-38. doi: 10.2217/imt.11.170. Immunotherapy. 2012. PMID: 22339463 Review.
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
-
Therapeutic potentials of plant iridoids in Alzheimer's and Parkinson's diseases: A review.Eur J Med Chem. 2019 May 1;169:185-199. doi: 10.1016/j.ejmech.2019.03.009. Epub 2019 Mar 8. Eur J Med Chem. 2019. PMID: 30877973 Review.
-
Aducanumab and Its Effects on Tau Pathology: Is This the Turning Point of Amyloid Hypothesis?Int J Mol Sci. 2022 Feb 11;23(4):2011. doi: 10.3390/ijms23042011. Int J Mol Sci. 2022. PMID: 35216126 Free PMC article. Review.
Cited by
-
Blood-Brain Barrier Dysfunction in Normal Aging and Neurodegeneration: Mechanisms, Impact, and Treatments.Stroke. 2023 Mar;54(3):661-672. doi: 10.1161/STROKEAHA.122.040578. Epub 2023 Feb 27. Stroke. 2023. PMID: 36848419 Free PMC article. Review.
-
Proteolysis-Targeting Chimera (PROTAC) Delivery into the Brain across the Blood-Brain Barrier.Antibodies (Basel). 2023 Jun 26;12(3):43. doi: 10.3390/antib12030043. Antibodies (Basel). 2023. PMID: 37489365 Free PMC article. Review.
-
Breaking Bad Proteins-Discovery Approaches and the Road to Clinic for Degraders.Cells. 2024 Mar 26;13(7):578. doi: 10.3390/cells13070578. Cells. 2024. PMID: 38607017 Free PMC article. Review.
-
Advancements and Challenges in Antiamyloid Therapy for Alzheimer's Disease: A Comprehensive Review.Int J Alzheimers Dis. 2024 Jul 23;2024:2052142. doi: 10.1155/2024/2052142. eCollection 2024. Int J Alzheimers Dis. 2024. PMID: 39081336 Free PMC article. Review.
-
Phosphorylated Tau in Alzheimer's Disease and Other Tauopathies.Int J Mol Sci. 2022 Oct 25;23(21):12841. doi: 10.3390/ijms232112841. Int J Mol Sci. 2022. PMID: 36361631 Free PMC article. Review.
References
-
- Jack C.R., Jr., Knopman D.S., Jagust W.J., Petersen R.C., Weiner M.W., Aisen P.S., Shaw L.M., Vemuri P., Wiste H.J., Weigand S.D., et al. Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

