Neutrophil dysfunction in the pathogenesis of cystic fibrosis
- PMID: 35213685
- PMCID: PMC9053701
- DOI: 10.1182/blood.2021014699
Neutrophil dysfunction in the pathogenesis of cystic fibrosis
Abstract
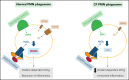
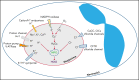
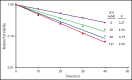
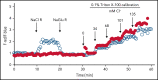
Polymorphonuclear neutrophils (PMNs) figure prominently in host defense against infection and in noninfectious inflammation. Mobilized early in an inflammatory response, PMNs mediate immediate cellular defense against microbes and orchestrate events that culminate in cessation of inflammation and restoration of homeostasis. Failure to terminate the inflammatory response and its causes can fuel exuberant inflammation characteristic of many human diseases, including cystic fibrosis (CF), an autosomal recessive genetic disease caused by mutations in the CF transmembrane conductance regulator. CF affects multiple end organs, with persistent bacterial infection and chronic neutrophilic inflammation in airways predominating the clinical picture. To match the diverse microbial challenges that they may encounter, PMNs possess a variety of antimicrobial systems to slow or kill invading microorganisms confined in their phagosomes. Prominent among PMN defense systems is their ability to generate hypochlorous acid, a potent microbicide, by reacting oxidants generated by the NADPH oxidase with myeloperoxidase (MPO) released from azurophilic granules in the presence of chloride (Cl-). Products of the MPO-H2O2-Cl system oxidize susceptible biomolecules and support robust antimicrobial action against many, but not all, potential human pathogens. Underscoring that the MPO-H2O2-Cl system is integral to optimal host defense and proper regulation of inflammation, individuals with defects in any component of this system, as seen in chronic granulomatous disease or MPO deficiency, incur increased rates or severity of infection and signs of dysregulated inflammatory responses. We focus attention in this review on the molecular basis for and the clinical consequences of defects in the MPO-H2O2-Cl system because of the compromised Cl transport seen in CF. We will discuss first how the MPO-H2O2-Cl system in healthy PMNs participates in host defense and resolution of inflammation and then review how a defective MPO-H2O2-Cl system contributes to the increased susceptibility to infection and dysregulated inflammation associated with the clinical manifestations of CF.
© 2022 by The American Society of Hematology.
Figures
Similar articles
-
Viricidal effect of polymorphonuclear leukocytes on human immunodeficiency virus-1. Role of the myeloperoxidase system.J Clin Invest. 1992 Jun;89(6):2014-7. doi: 10.1172/JCI115810. J Clin Invest. 1992. PMID: 1318327 Free PMC article.
-
The role of chloride anion and CFTR in killing of Pseudomonas aeruginosa by normal and CF neutrophils.J Leukoc Biol. 2008 Jun;83(6):1345-53. doi: 10.1189/jlb.0907658. Epub 2008 Mar 19. J Leukoc Biol. 2008. PMID: 18353929 Free PMC article.
-
Substrate-dependent metabolomic signatures of myeloperoxidase activity in airway epithelial cells: Implications for early cystic fibrosis lung disease.Free Radic Biol Med. 2023 Sep;206:180-190. doi: 10.1016/j.freeradbiomed.2023.06.021. Epub 2023 Jun 23. Free Radic Biol Med. 2023. PMID: 37356776 Free PMC article.
-
Salt, chloride, bleach, and innate host defense.J Leukoc Biol. 2015 Aug;98(2):163-72. doi: 10.1189/jlb.4RU0315-109R. Epub 2015 Jun 5. J Leukoc Biol. 2015. PMID: 26048979 Free PMC article. Review.
-
Myeloperoxidase: a front-line defender against phagocytosed microorganisms.J Leukoc Biol. 2013 Feb;93(2):185-98. doi: 10.1189/jlb.0712349. Epub 2012 Oct 11. J Leukoc Biol. 2013. PMID: 23066164 Free PMC article. Review.
Cited by
-
Impact of CFTR Modulators on the Impaired Function of Phagocytes in Cystic Fibrosis Lung Disease.Int J Mol Sci. 2022 Oct 17;23(20):12421. doi: 10.3390/ijms232012421. Int J Mol Sci. 2022. PMID: 36293274 Free PMC article. Review.
-
Nanoparticle-neutrophils interactions for autoimmune regulation.Adv Drug Deliv Rev. 2024 Jun;209:115316. doi: 10.1016/j.addr.2024.115316. Epub 2024 Apr 23. Adv Drug Deliv Rev. 2024. PMID: 38663550 Review.
-
Cftr deletion in mouse epithelial and immune cells differentially influence the intestinal microbiota.Commun Biol. 2022 Oct 26;5(1):1130. doi: 10.1038/s42003-022-04101-5. Commun Biol. 2022. PMID: 36289287 Free PMC article.
-
Approaches for neutrophil imaging: an important step in personalized medicine.Bioengineered. 2022 Jun;13(6):14844-14855. doi: 10.1080/21655979.2022.2096303. Bioengineered. 2022. PMID: 36469646 Free PMC article. Review.
-
Lytic bacteriophages induce the secretion of antiviral and proinflammatory cytokines from human respiratory epithelial cells.PLoS Biol. 2024 Apr 23;22(4):e3002566. doi: 10.1371/journal.pbio.3002566. eCollection 2024 Apr. PLoS Biol. 2024. PMID: 38652717 Free PMC article.
References
-
- Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33(5):657-670. - PubMed
-
- Rankin SM. The bone marrow: a site of neutrophil clearance. J Leukoc Biol. 2010;88(2):241-251. - PubMed
-
- Nauseef WM. How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev. 2007;219(1):88-102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

