Natriuretic Peptide Oligomers Cause Proarrhythmic Metabolic and Electrophysiological Effects in Atrial Myocytes
- PMID: 35212578
- PMCID: PMC8930702
- DOI: 10.1161/CIRCEP.121.010636
Natriuretic Peptide Oligomers Cause Proarrhythmic Metabolic and Electrophysiological Effects in Atrial Myocytes
Abstract
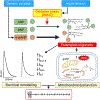
Background: With aging, the human atrium invariably develops amyloid composed of ANP (atrial natriuretic peptide) and BNP (B-type natriuretic peptide). Preamyloid oligomers are the primary cytotoxic species in amyloidosis, and they accumulate in the atrium during human hypertension and a murine hypertensive model of atrial fibrillation susceptibility. We tested the hypothesis that preamyloid oligomers derived from natriuretic peptides cause cytotoxic and electrophysiological effects in atrial cells that promote arrhythmia susceptibility and that oligomer formation is enhanced for a mutant form of ANP linked to familial atrial fibrillation.
Methods: Oligomerization was assessed by Western blot analysis. Bioenergic profiling was performed using the Seahorse platform. Mitochondrial dynamics were investigated with immunostaining and gene expression quantitated using quantitative reverse transcription polymerase chain reaction. Action potentials and ionic currents were recorded using patch-clamp methods and intracellular calcium measured using Fura-2.
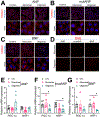
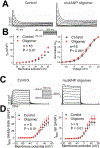
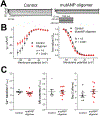
Results: Oligomer formation was markedly accelerated for mutant ANP (mutANP) compared with WT (wild type) ANP. Oligomers derived from ANP, BNP, and mutANP suppressed mitochondrial function in atrial HL-1 cardiomyocytes, associated with increased superoxide generation and reduced biogenesis, while monomers had no effects. In hypertensive mice, atrial cardiomyocytes displayed reduced action potential duration and maximal dV/dT of phase 0, with an elevated resting membrane potential, compared with normotensive mice. Similar changes were observed when atrial cells were exposed to oligomers. mutANP monomers produced similar electrophysiological effects as mutANP oligomers, likely due to accelerated oligomer formation, while ANP and BNP monomers did not. Oligomers decreased Na+ current, inward rectifier K+ current, and L-type Ca++ current, while increasing sustained and transient outward K+ currents, to account for these effects.
Conclusions: These findings provide compelling evidence that natriuretic peptide oligomers are novel mediators of atrial arrhythmia susceptibility. Moreover, the accelerated oligomerization by mutANP supports a role for these mediators in the pathophysiology of this mutation in atrial fibrillation.
Keywords: atrial fibrillation; atrial natriuretic factor; calcium; electrophysiology; natriuretic peptide, brain.
Figures




Similar articles
-
Effects of Wild-Type and Mutant Forms of Atrial Natriuretic Peptide on Atrial Electrophysiology and Arrhythmogenesis.Circ Arrhythm Electrophysiol. 2015 Oct;8(5):1240-54. doi: 10.1161/CIRCEP.115.002896. Epub 2015 Jul 30. Circ Arrhythm Electrophysiol. 2015. PMID: 26227000
-
Electrophysiologic and molecular mechanisms of a frameshift NPPA mutation linked with familial atrial fibrillation.J Mol Cell Cardiol. 2019 Jul;132:24-35. doi: 10.1016/j.yjmcc.2019.05.004. Epub 2019 May 8. J Mol Cell Cardiol. 2019. PMID: 31077706 Free PMC article.
-
B-Type Natriuretic Peptide Modulates Pulmonary Vein Arrhythmogenesis: A Novel Potential Contributor to the Genesis of Atrial Tachyarrhythmia in Heart Failure.J Cardiovasc Electrophysiol. 2016 Dec;27(12):1462-1471. doi: 10.1111/jce.13093. Epub 2016 Oct 3. J Cardiovasc Electrophysiol. 2016. PMID: 27571932
-
Cardiac natriuretic peptides.Nat Rev Cardiol. 2020 Nov;17(11):698-717. doi: 10.1038/s41569-020-0381-0. Epub 2020 May 22. Nat Rev Cardiol. 2020. PMID: 32444692 Review.
-
Atrial and brain natriuretic peptides: Hormones secreted from the heart.Peptides. 2019 Jan;111:18-25. doi: 10.1016/j.peptides.2018.05.012. Epub 2018 May 31. Peptides. 2019. PMID: 29859763 Review.
Cited by
-
Heart Failure with Preserved Ejection Fraction and Cardiac Amyloidosis in the Aging Heart.Int J Mol Sci. 2024 Oct 26;25(21):11519. doi: 10.3390/ijms252111519. Int J Mol Sci. 2024. PMID: 39519069 Free PMC article. Review.
-
Isolevuglandins Promote Mitochondrial Dysfunction and Electrophysiologic Abnormalities in Atrial Cardiomyocytes.Cells. 2024 Mar 9;13(6):483. doi: 10.3390/cells13060483. Cells. 2024. PMID: 38534327 Free PMC article.
-
Modulation of NOX2 causes obesity-mediated atrial fibrillation.J Clin Invest. 2024 Aug 15;134(18):e175447. doi: 10.1172/JCI175447. J Clin Invest. 2024. PMID: 39146015 Free PMC article.
-
Atrial cardiomyopathy revisited-evolution of a concept: a clinical consensus statement of the European Heart Rhythm Association (EHRA) of the ESC, the Heart Rhythm Society (HRS), the Asian Pacific Heart Rhythm Society (APHRS), and the Latin American Heart Rhythm Society (LAHRS).Europace. 2024 Aug 30;26(9):euae204. doi: 10.1093/europace/euae204. Europace. 2024. PMID: 39077825 Free PMC article.
-
Atrial amyloidosis: mechanisms and clinical manifestations.Eur J Heart Fail. 2022 Nov;24(11):2019-2028. doi: 10.1002/ejhf.2650. Epub 2022 Aug 21. Eur J Heart Fail. 2022. PMID: 35920110 Free PMC article. Review.
References
-
- Willis MS and Patterson C. Proteotoxicity and cardiac dysfunction--Alzheimer's disease of the heart? N Engl J Med. 2013;368:455–464. - PubMed
-
- Guerrero-Munoz MJ, Castillo-Carranza DL and Kayed R. Therapeutic approaches against common structural features of toxic oligomers shared by multiple amyloidogenic proteins. Biochem Pharmacol. 2014;88:468–478. - PubMed
-
- Rocken C, Peters B, Juenemann G, Saeger W, Klein HU, Huth C, Roessner A and Goette A. Atrial amyloidosis: an arrhythmogenic substrate for persistent atrial fibrillation. Circulation. 2002;106:2091–2097. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

