Amyloidosis in Alzheimer's Disease: Pathogeny, Etiology, and Related Therapeutic Directions
- PMID: 35209007
- PMCID: PMC8876037
- DOI: 10.3390/molecules27041210
Amyloidosis in Alzheimer's Disease: Pathogeny, Etiology, and Related Therapeutic Directions
Abstract
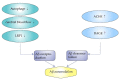
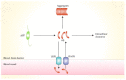
The amyloid hypothesis of Alzheimer's disease has long been the predominant theory, suggesting that Alzheimer's disease is caused by the accumulation of amyloid beta protein (Aβ) in the brain, leading to neuronal toxicity in the central nervous system (CNS). Because of breakthroughs in molecular medicine, the amyloid pathway is thought to be central to the pathophysiology of Alzheimer's disease (AD). Currently, it is believed that altered biochemistry of the Aβ cycle remains a central biological feature of AD and is a promising target for treatment. This review provides an overview of the process of amyloid formation, explaining the transition from amyloid precursor protein to amyloid beta protein. Moreover, we also reveal the relationship between autophagy, cerebral blood flow, ACHE, expression of LRP1, and amyloidosis. In addition, we discuss the detailed pathogenesis of amyloidosis, including oxidative damage, tau protein, NFTs, and neuronal damage. Finally, we list some ways to treat AD in terms of decreasing the accumulation of Aβ in the brain.
Keywords: Alzheimer’s disease; amyloid beta protein; amyloid precursor protein; amyloidosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Loss of LR11/SORLA enhances early pathology in a mouse model of amyloidosis: evidence for a proximal role in Alzheimer's disease.J Neurosci. 2008 Nov 26;28(48):12877-86. doi: 10.1523/JNEUROSCI.4582-08.2008. J Neurosci. 2008. PMID: 19036982 Free PMC article.
-
A{beta} accelerates the spatiotemporal progression of tau pathology and augments tau amyloidosis in an Alzheimer mouse model.Am J Pathol. 2010 Oct;177(4):1977-88. doi: 10.2353/ajpath.2010.100346. Epub 2010 Aug 27. Am J Pathol. 2010. PMID: 20802182 Free PMC article.
-
Understanding the Amyloid Hypothesis in Alzheimer's Disease.J Alzheimers Dis. 2019;68(2):493-510. doi: 10.3233/JAD-180802. J Alzheimers Dis. 2019. PMID: 30883346 Review.
-
Diversity in Aβ deposit morphology and secondary proteome insolubility across models of Alzheimer-type amyloidosis.Acta Neuropathol Commun. 2020 Apr 6;8(1):43. doi: 10.1186/s40478-020-00911-y. Acta Neuropathol Commun. 2020. PMID: 32252825 Free PMC article.
-
Abeta as a bioflocculant: implications for the amyloid hypothesis of Alzheimer's disease.Neurobiol Aging. 2002 Nov-Dec;23(6):1051-72. doi: 10.1016/s0197-4580(01)00342-6. Neurobiol Aging. 2002. PMID: 12470802 Review.
Cited by
-
Impaired Mitochondrial Energy Metabolism Regulated by p70S6K: A Putative Pathological Feature in Alzheimer's Disease.Metabolites. 2024 Jun 29;14(7):369. doi: 10.3390/metabo14070369. Metabolites. 2024. PMID: 39057692 Free PMC article.
-
Naringin and Naringenin: Potential Multi-Target Agents for Alzheimer's Disease.Curr Med Sci. 2024 Oct;44(5):867-882. doi: 10.1007/s11596-024-2921-z. Epub 2024 Sep 30. Curr Med Sci. 2024. PMID: 39347923 Review.
-
Multidimensional autophagy nano-regulator boosts Alzheimer's disease treatment by improving both extra/intraneuronal homeostasis.Acta Pharm Sin B. 2024 Mar;14(3):1380-1399. doi: 10.1016/j.apsb.2023.10.009. Epub 2023 Oct 21. Acta Pharm Sin B. 2024. PMID: 38486986 Free PMC article.
-
Modifying Alzheimer's disease pathophysiology with photobiomodulation: model, evidence, and future with EEG-guided intervention.Front Neurol. 2024 Aug 23;15:1407785. doi: 10.3389/fneur.2024.1407785. eCollection 2024. Front Neurol. 2024. PMID: 39246604 Free PMC article. Review.
-
Nanotechnology-based drug delivery for the treatment of CNS disorders.Transl Neurosci. 2022 Dec 31;13(1):527-546. doi: 10.1515/tnsci-2022-0258. eCollection 2022 Jan 1. Transl Neurosci. 2022. PMID: 36741545 Free PMC article. Review.
References
-
- Prince M.J., Wimo A., Guerchet M.M., Ali G.C., Wu Y.-T., Prina M. World Alzheimer Report 2015—The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends. Volume 2015. Alzheimer’s Disease International; London, UK: 2015. p. 84.
-
- Hohenfeld C., Kuhn H., Muller C., Nellessen N., Ketteler S., Heinecke A., Goebel R., Shah N.J., Schulz J.B., Reske M., et al. Changes in brain activation related to visuo-spatial memory after real-time fMRI neurofeedback training in healthy elderly and Alzheimer’s disease. Behav. Brain Res. 2020;381:112435. doi: 10.1016/j.bbr.2019.112435. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous